AbstractPurposeCancer cells frequently express genes that are specifically or preferentially expressed in male germ cells under normal conditions. The ATPase family AAA domain-containing 2 (ATAD2) is one such and works as an important cofactor for MYC-dependent transcription. In hepatocellular carcinoma (HCC), ATAD2 has been identified as a candidate driver gene located within the amplified 8q24 locus. However, the prognostic significance of ATAD2 protein expression in HCC remains uncertain.
Materials and MethodsWe investigated ATAD2 protein expression by immunohistochemistry in tumor tissue from 182 HCC patients who underwent curative resection. Associations of ATAD2 expression with clinicopathologic variables or prognosis of HCC patients were analyzed.
ResultsATAD2 expression was observed in 119 (65.4%) of the 182 HCCs and tended to be independent predictor of early recurrence (p=0.059). ATAD2 expression showed an unfavorable influence on recurrence-free survival (RFS) (p < 0.001). Subgroup analysis among patients with tumor size ≤ 5.0 cm (n=109), patients at Barcelona Clinic Liver Cancer stage 0 or A (n=92), and patients with α-fetoprotein ≤ 20 ng/mL (n=61), the ATAD2-positive groups unfavorably influenced RFS (p=0.008, p=0.009, and p=0.013, respectively). In addition, ATAD2 expression was an independent predictor of shorter RFS (p=0.002). ATAD2 expression showed an unfavorable influence on disease-specific survival (p=0.001), but was not an independent predictor of shorter disease-specific survival (p=0.109).
IntroductionHepatocellular carcinoma (HCC) is one of the most lethal malignancies, and surgical resection improves the survival rates for patients. However, the prognosis after surgical resection of HCC remains poor because of high recurrence rates [1,2]. Using biomarkers to identify patients presenting with a higher risk of poor prognosis may reduce mortality after curative hepatectomy. Although there are many reports on histologic parameters for predicting HCC prognosis, molecular markers for HCC recurrence and prognosis could provide additional information [3].
Cancer cells frequently express genes that are specifically or preferentially expressed in male germ cells under normal conditions [4]. ATPase family AAA domain-containing 2 (ATAD2) is one such, and works as an important cofactor for MYC-dependent transcription [5]. Through MYC and E2F transcription factors, ATAD2 increases the expression of proliferation-related and anti-apoptotic genes in many different types of cancer, including breast carcinoma, non-small cell lung carcinoma, and prostate carcinoma [5-8]. High ATAD2 is associated with poor survival of patients with breast cancer [5,6]. In HCC, ATAD2 has been identified as a candidate driver gene located within the amplified 8q24 locus [9]. Huang et al. [10] reported that a novel highly up-regulated exon-exon junction was detected in ATAD2 gene by RNA-seq and the gene was highly expressed in HCC tissues. A recent study found that the high expression of ATAD2 in HCC was an independent predictor of shortened overall survival [11]. However, the prognostic significance of ATAD2 protein expression in HCC is unclear.
In the present study, we evaluated ATAD2 protein expression by immunohistochemistry to elucidate the prognostic role of ATAD2 in 182 HCC patients with long-term follow-up.
Materials and Methods1. Tissue samplesHCC tissue samples were collected from 182 patients who were treated with surgical resection between July 2000 and May 2006 at Samsung Medical Center (Seoul, Korea). Inclusion criteria were histologically confirmed HCC and curative resection of tumor without preoperative or postoperative adjuvant therapy. We defined curative resection as complete resection of all tumor nodules with clear microscopic resection margins and no residual tumors as indicated by a computed tomography scan one month after surgery. The Institutional Review Board of Samsung Medical Center approved this study. Tumor differentiation was graded histologically following Edmondson and Steiner criteria [12]. Microvascular invasion was considered present when at least one or more endothelial cells or the tunica media of the vessel surrounded a neoplastic cell group. Intrahepatic metastasis and multicentric occurrence were defined according to previously reported criteria [13]. Tumor stage was determined by both the American Joint Committee on Cancer (AJCC) staging system [14] and Barcelona Clinic Liver Cancer (BCLC) staging classification [15]. Using 2 years as the cut off, tumor recurrence was classified as either early recurrence or late recurrence [16,17].
All HCC patients were followed by monitoring serum α-fetoprotein levels and three phase dynamic computed tomography scans or magnetic resonance imaging every three months after surgery. The follow-up period for recurrence was at least 18 months, and the median follow-up period was 120.0 months (range, 14.0 to 151.4 months) for survivors. Recurrence-free survival (RFS) was defined as from the date of resection until the detection of tumor recurrence. We chose disease-specific death (HCC-related death) as the clinical endpoint for survival analysis, defined as: (1) tumor occupying more than 80% of the liver, (2) portal venous tumor thrombus proximal to the second bifurcation, (3) obstructive jaundice due to the tumor, (4) distant metastases, or (5) variceal hemorrhage with portal venous tumor thrombus proximal to the first bifurcation [18]. Disease-specific survival (DSS) was defined from the date of resection to the date of HCC-related death.
Histologic sections were examined by two pathologists and representative tumor regions were marked in the formalin-fixed paraffin-embedded blocks. Two tissue cores measuring 2.0 mm in diameter were punched from the marked areas of each block and arranged into new paraffin blocks. As controls, two cores of normal liver tissue from 12 patients with metastatic colon carcinoma of the liver were included in each array block.
2. Immunohistochemical analysisImmunohistochemical staining was performed with standard methods [19]. Antigen retrieval was performed with 0.01 mol/L citrate buffer at pH 6.0 for 30 minutes in a pressure cooker. Sections were incubated with a rabbit polyclonal antibody to ATAD2 (1:100, NBP1-84122, Novus Biologicals, Littleton, CO) for 60 minutes at room temperature. The positive control (human normal testis) showed intense nuclear ATAD2 expression in spermatogenic cells of seminiferous tubules. No immunoreactivity was observed in tissue sections used as negative control where the primary antibody was replaced by preimmune rabbit serum. In order to validate the concordance between tissue microarrays and whole tumor sections, we observed ATAD2 expression for 40 corresponding whole tumor sections randomly chosen from the 182 cases.
All sections were scored by two independent pathologists (C.-K.P. and H.W.H.) who were blinded to the clinical details, and any discrepancies were resolved by consensus. A nearly homogeneous nuclear immunostaining with moderate staining intensity was observed. For determining ATAD2 expression, the proportion of stained tumor cells was determined semi-quantitatively and each sample was scored on a scale of 0-4 (0, < 5%; 1, 5%-25%; 2, 26%-50%; 3, 51%-75%; 4, > 75%). Duplicate tissue cores for each tumor showed high levels of homogeneity for proportion of stained cells. When there were differences between duplicate tissue cores, the higher score was taken.
3. Statistical analysisStatistical analyses were performed with SPSS ver. 18 software (SPSS Inc., Chicago, IL). The association of ATAD2 expression with clinicopathologic features was examined by the chi-square test or Fisher exact test. Univariate and multivariate analyses of risks for tumor recurrence were performed using the logistic regression model. Cumulative survival rates were calculated by the Kaplan-Meier method and analyzed by the log-rank test. The Cox proportional hazards regression model was used to assess the association of survival time regressed upon multiple clinicopathologic variables. Variables that were statistically significant in the univariate analysis were included in the multivariate analysis. A p-values less than 0.05 were considered to be statistically significant.
Results1. Patient characteristicsThe mean age of the 182 HCC patents was 52.4 years (range, 17 to 76 years), and 82.4% of the patients were male. Chronic hepatitis B virus infection was detected in 141 patients (77.5%) and chronic hepatitis C virus infection in 16 patients (8.8%). No viral marker was recognized in 25 patients (13.7%). The mean tumor size was 5.3 cm, and 109 of the 182 tumors (59.9%) were ≤ 5 cm in size. Tumor recurrence was detected in 132 patients (72.5%), early recurrence in 108 patients (59.3%), and late recurrence in 24 patients (13.2%). Seventy-six patients (41.8 %) died of HCC. Fifteen of the 91 deaths in this study were due to non-HCC causes. Nine of the 15 deaths were due to hepatic failure, five due to non-hepatic causes, and one due to unknown cause.
2. ATAD2 protein expression in HCCATAD2 protein was rarely detected on the nucleus of normal hepatocytes. In HCC, immunoreactivity for ATAD2 was observed only in the nuclei of tumor cells with moderate staining intensity. We regarded the ATAD2 as positive when ≥ 5% of tumor cells showed nuclear immunoreactivity (Fig. 1). ATAD2 protein expression was observed in 119 of the 182 HCCs (65.4%). ATAD2 expression was significantly associated with larger tumor size (p=0.001), higher Edmondson grade (p=0.015), microvascular invasion (p < 0.001), intrahepatic metastasis (p < 0.001), higher AJCC T-stage (p < 0.001), and higher BCLC stage (p=0.004). ATAD2 expression was associated with the early recurrence (p < 0.001), but not with the late recurrence (p=0.619) (Table 1).
3. Prediction of early recurrence in HCCIn univariate analyses, early recurrence was significantly associated with larger tumor size (p=0.019), microvascular invasion (p < 0.001), intrahepatic metastasis (p < 0.001), higher AJCC T-stage (p < 0.001), higher BCLC stage (p < 0.001), lower albumin level (p=0.041), viral etiology (p=0.004), and ATAD2 expression (p < 0.001). As AJCC T-stage and BCLC stage were associated with vascular invasion, we did not make multiple analyses with these variables to avoid potential bias. An evaluation of the association of serum α-fetoprotein level with early recurrence was not performed due to missing data (n=175). In multivariate analyses, microvascular invasion (p=0.003), intrahepatic metastasis (p=0.003), and viral etiology (p=0.029) were independent predictors of early recurrence. ATAD2 expression tended to be independent predictor of early recurrence (p=0.059) (Table 2).
4. Association between ATAD2 expression and prognosis of HCC patientsThe RFS and DSS rates for 182 HCC patients were 36.1% and 75.5% at 3 years, 32.1% and 66.7% at 5 years, 27.5% and 61.1% at 7 years, and 26.6% and 55.2% at 9 years, respectively. On univariate analyses, larger tumor size, microvascular invasion, major portal vein invasion, intrahepatic metastasis, higher AJCC T-stage, higher BCLC stage, and lower albumin level showed unfavorable influences on both RFS and DSS. Higher α-fetoprotein level and viral etiology unfavorably influenced RFS. ATAD2 expression unfavorably influenced RFS (p < 0.001) (Table 3). The 5-year RFS rate of the ATAD2-positive group was significantly lower than that of the ATAD2-negative group (23.1% vs. 48.6%) (Fig. 2A). The mean RFS of ATAD2-positive group was 37.6 months and the ATAD2-negative group was 74.0 months. Subgroup analysis among patients with tumor size ≤ 5.0 cm (n=109), patients at BCLC stage 0 or A (n=92), and patients with α-fetoprotein ≤ 20 ng/mL (n=61), the ATAD2-positive groups (n=61, n=50, and n=30, respectively) also unfavorably influenced RFS (p=0.008, p=0.009, and p=0.013, respectively) (Fig. 2B, C, and D).
ATAD2 expression unfavorably influenced DSS (p=0.001) (Table 3). The 5-year DSS rate of the ATAD2-positive group was significantly lower than that of the ATAD2-negative group (57.8% vs. 83.4%) (Fig. 3). The mean DSS of ATAD2-positive group was 88.1 months and of ATAD2-negative group, 123.3 months.
Multivariate analyses indicated that intrahepatic metastasis and lower albumin level were found to be independent predictors of both shorter RFS and shorter DSS. Viral etiology was an independent predictor of shorter RFS. ATAD2 expression (p=0.002) was an independent predictor of shorter RFS, but not of DSS (p=0.109). ATAD2-positive patients were more likely to suffer from recurrence than ATAD2-negative patients (hazard ratio, 1.857) (Table 4).
DiscussionATAD2 contributes to the development of aggressive cancer through the enhancement of MYC-dependent transcription [5]. ATAD2 expression is high in several human cancers, including breast carcinoma, colon carcinoma, lung carcinoma, stomach carcinoma, and uterine carcinoma [5]. High ATAD2 expression is a strong predictor of rapid mortality in lung and breast cancers [7]. ATAD2 depletion by RNA interference knockdown reduced HUH7 and HCCLM3 cell proliferation and led to a G1 phase cell cycle arrest [11]. In HCC cells, ATAD2 regulates cell migration/invasion via the regulation of APC and/or CTNNA1 expression [11]. In this study, we evaluated the prognostic significance of ATAD2 protein expression in HCC and demonstrated that ATAD2 expression is significantly associated with larger tumor size, higher Edmondson grade, microvascular invasion, intrahepatic metastasis, higher AJCC T-stage, and higher BCLC stage. Moreover, ATAD2 expression is an independent predictor of shorter RFS and tended to predict early recurrence.
In the present study, early stage HCC patients with tumor size ≤ 5.0 cm or patients at BCLC stage 0 or A have favorable outcomes in both RFS and DSS (Table 3), but 75 of 109 (68.8%) with tumor size ≤ 5.0 cm and 59 of 92 (64.1%) BCLC stage 0 or A developed tumor recurrence. We found within these populations, ATAD2 positivity unfavorably influenced RFS (p=0.008 and p=0.009, respectively). Identification of patients with poor prognosis in early stage HCC is critical to the optimization of personalized treatment. Thus, our findings support the possible importance of ATAD2 expression in HCC in detecting a phenotype that can predict recurrence risk in early stage HCC.
Serum α-fetoprotein is widely used to screen, mainly for HCC, and is an important predictor of patient survival following tumor resection [20]. Preoperative high serum α-fetoprotein level (> 60 ng/mL) could be a risk factor for recurrence after resection in patients with liver cirrhosis [21]. However, there is no effective marker for monitoring recurrence for patients with normal serum α-fetoprotein level (≤ 20 ng/mL) after hepatectomy. In this study, tumor recurrence was detected in 40 of 61 patients (65.6%) with normal serum α-fetoprotein level. We found that within patients with normal serum α-fetoprotein level, the ATAD2-positive group still had significantly poorer RFS than the ATAD2-negative group (p=0.013). Thus, our study revealed the potential use of ATAD2 in predicting recurrence risk in the patient group in which serum α-fetoprotein levels were not prognostically predictive.
ConclusionOur findings indicate that ATAD2 protein may be a novel potential predictor of RFS in HCC patients after curative resection, and ATAD2 may have prognostic value in HCC patients with early stage HCC or normal serum α-fetoprotein level. Further study is needed to examine the roles of ATAD2 protein expression in the development and progression of HCC.
Fig. 1.Immunostaining of ATAD2 showing positive nuclear expression (A) or negative expression (B) in hepatocellular carcinomas, no immunoreactivity in normal hepatocytes (C), and intense nuclear expression in spermatogenic cells of seminiferous tubules in normal human testis (D) (horseradish peroxidase staining, ×200). ATAD2, ATPase family AAA domain-containing 2. 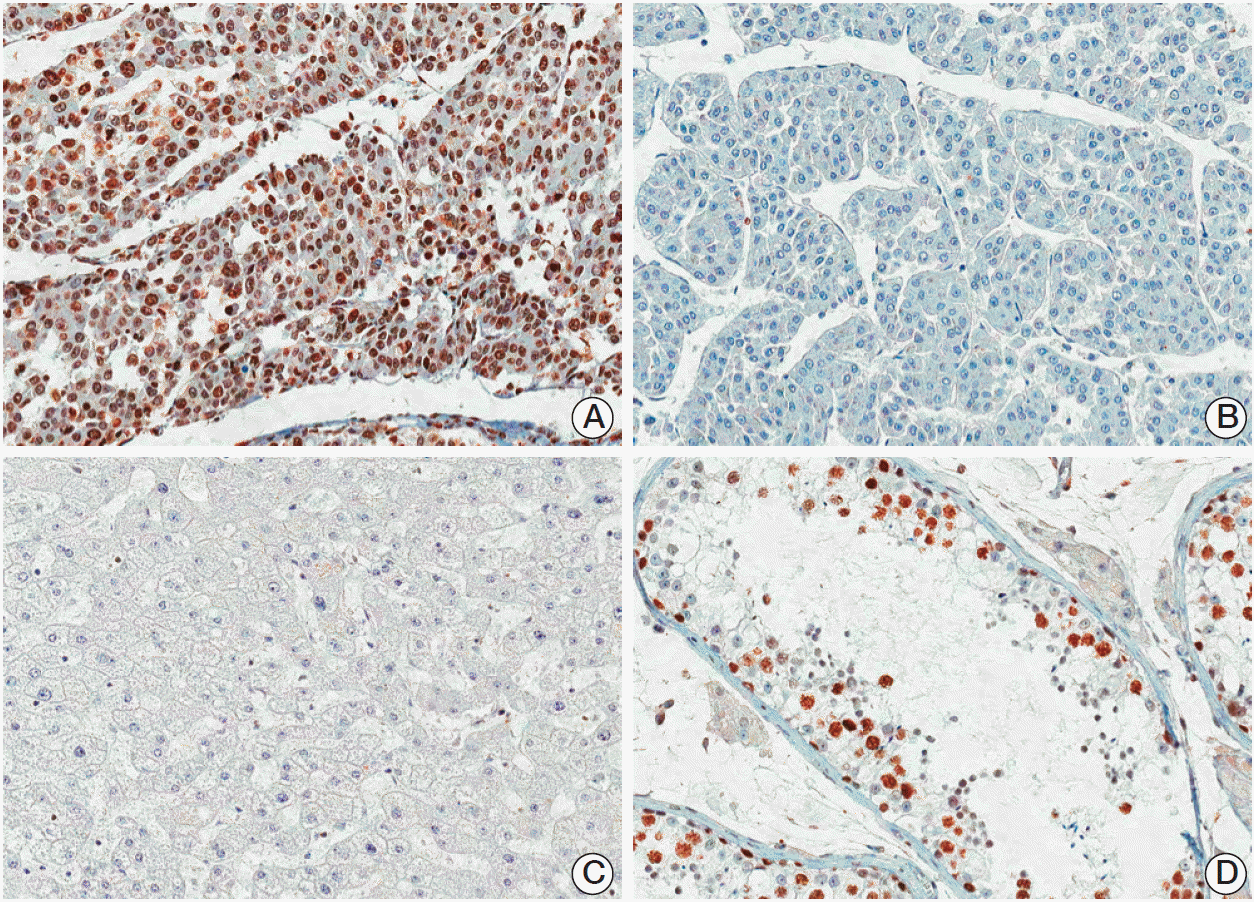
Fig. 2.Kaplan-Meier survival curves showing recurrence-free survival among all patients (A), patients with tumor size ≤ 5.0 cm (B), patients at BCLC stage 0 or A (C), and patients with AFP ≤ 20 ng/mL (D) according to the ATAD2 expression. BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; ATAD2, ATPase family AAA domain-containing 2. 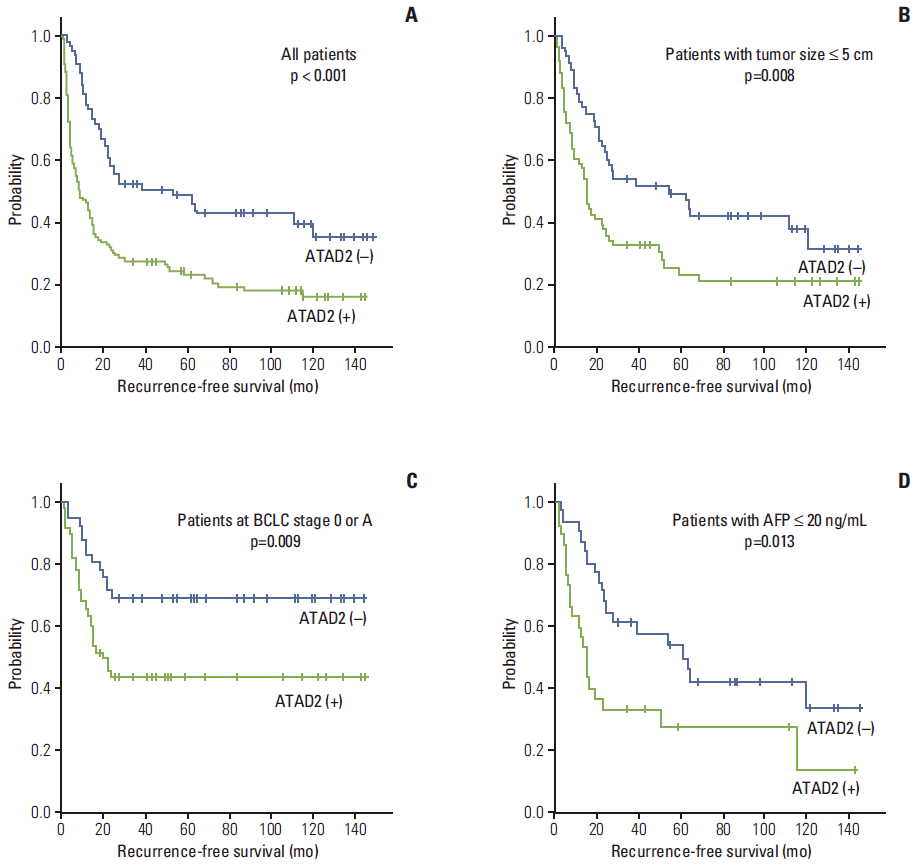
Fig. 3.Kaplan-Meier survival curves showing diseasespecific survival for ATAD2 expression in 182 hepatocellular carcinomas. ATAD2, ATPase family AAA domain-containing 2. 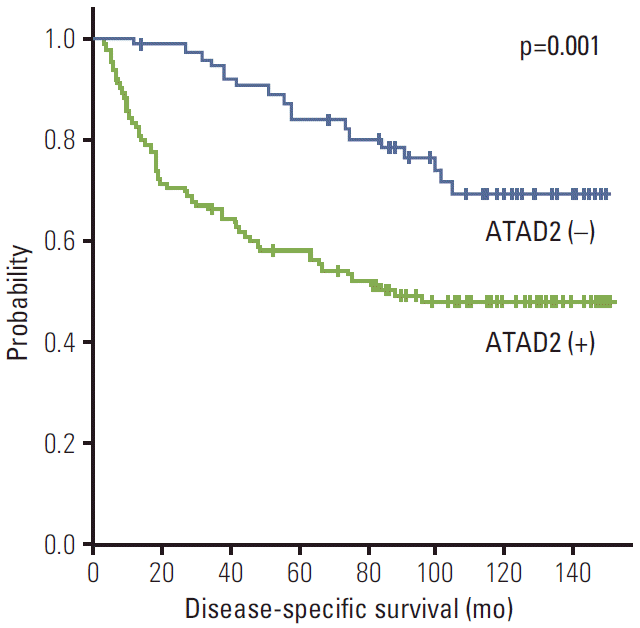
Table 1.ATAD2 expression and clinicopathologic features
in 182 hepatocellular carcinomas
Table 2.Prediction of early tumor recurrence in 182 hepatocellular carcinomas
Table 3.Univariate analyses of recurrence-free survival and disease-specific survival in 182 hepatocellular carcinomas
Table 4.Multivariate analyses of recurrence-free survival and disease-specific survival in 182 hepatocellular carcinomas References1. Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485–91.
2. Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology. 2011;54:757–9.
3. Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497–513.
4. Rousseaux S, Khochbin S. New hypotheses for large-scale epigenome alterations in somatic cancer cells: a role for male germ-cell-specific regulators. Epigenomics. 2009;1:153–61.
5. Ciro M, Prosperini E, Quarto M, Grazini U, Walfridsson J, McBlane F, et al. ATAD2 is a novel cofactor for MYC, overexpressed and amplified in aggressive tumors. Cancer Res. 2009;69:8491–8.
6. Kalashnikova EV, Revenko AS, Gemo AT, Andrews NP, Tepper CG, Zou JX, et al. ANCCA/ATAD2 overexpression identifies breast cancer patients with poor prognosis, acting to drive proliferation and survival of triple-negative cells through control of B-Myb and EZH2. Cancer Res. 2010;70:9402–12.
7. Caron C, Lestrat C, Marsal S, Escoffier E, Curtet S, Virolle V, et al. Functional characterization of ATAD2 as a new cancer/testis factor and a predictor of poor prognosis in breast and lung cancers. Oncogene. 2010;29:5171–81.
8. Zou JX, Guo L, Revenko AS, Tepper CG, Gemo AT, Kung HJ, et al. Androgen-induced coactivator ANCCA mediates specific androgen receptor signaling in prostate cancer. Cancer Res. 2009;69:3339–46.
9. Wang K, Lim HY, Shi S, Lee J, Deng S, Xie T, et al. Genomic landscape of copy number aberrations enables the identification of oncogenic drivers in hepatocellular carcinoma. Hepatology. 2013;58:706–17.
10. Huang Q, Lin B, Liu H, Ma X, Mo F, Yu W, et al. RNA-Seq analyses generate comprehensive transcriptomic landscape and reveal complex transcript patterns in hepatocellular carcinoma. PLoS One. 2011;6:e26168
11. Wu G, Liu H, He H, Wang Y, Lu X, Yu Y, et al. miR-372 downregulates the oncogene ATAD2 to influence hepatocellular carcinoma proliferation and metastasis. BMC Cancer. 2014;14:107.
12. Edmondson HA, Liu H, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503.
13. Kumada T, Nakano S, Takeda I, Sugiyama K, Osada T, Kiriyama S, et al. Patterns of recurrence after initial treatment in patients with small hepatocellular carcinoma. Hepatology. 1997;25:87–92.
14. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th edNew York: Springer; 2010.
15. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38.
16. Shimada M, Hamatsu T, Yamashita Y, Rikimaru T, Taguchi K, Utsunomiya T, et al. Characteristics of multicentric hepatocellular carcinomas: comparison with intrahepatic metastasis. World J Surg. 2001;25:991–5.
17. Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–7.
18. Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;35:1995–2004.
19. Ahn S, Hyeon J, Park CK. Metadherin is a prognostic predictor of hepatocellular carcinoma after curative hepatectomy. Gut Liver. 2013;7:206–12.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||