AbstractPurposeUp to 10% of recurrences develop beyond 5 years after curative treatment of localized renal cell carcinoma (RCC). Clinicopathologic features were evaluated to determine which factors are associated with late recurrence.
Materials and MethodsA total of 753 patients were diagnosed with localized RCC from January 2000 to June 2008. We enrolled 225 patients who were treated surgically and had a minimal recurrence-free survival of 60 months. Patients who had recurrence beyond 5 years after nephrectomy were defined as the late recurrence group and the remaining patients as the recurrence-free group. Multivariate logistic regression analyses and the Cox proportional hazard model were used for determination of features associated with late recurrence.
ResultsIn multivariate analyses, age older than 60 (p=0.030), Fuhrman grade ≥ 3 (p=0.042), and pT stage ≥ pT2 (p=0.010) showed statistical association with late recurrence. The Cox proportional hazard model showed significant differences in recurrence-free survival when we classified the patients based on pT2 (p=0.007) and on patient age ≥ 60 years (p=0.039).
IntroductionKidney cancer is the second most common urologic tumor, with 3,598 new cases reported in Korea in 2010 [1]. According to current guidelines, radical surgery remains the only curative approach for patients with localized renal cell carcinoma (RCC) [2-4]. As imaging modalities have improved, detection of small renal masses has become much easier and many patients can receive appropriate treatment, including nephron-sparing surgery [5]. As a result, 5-year and 10-year survival rates have improved during the last two decades. However, development of disease recurrence after adequately performed nephrectomy has been reported in 20%-40% of patients with localized RCC [6]. Although recurrences usually develop within the first 3 to 5 years after surgical treatment, approximately 10% of patients show recurrence more than 5 years after initial nephrectomy [7,8]. Therefore, most clinicians hesitate to stop follow-up for their RCC patients, although many guidelines state that follow-up is not necessary for RCC patients who have no relapse for more than 5 years after surgery.
Many studies have attempted to predict the recurrence of RCC and it is now known that tumor size, tumor histology, and pathologic stage are factors associated with disease recurrence. Based on these findings, several nomograms have been developed for evaluation of the risk of metastasis or disease recurrence [9-11]. However, the risk of late recurrence cannot be calculated using these nomograms, and clinical characteristics and predictive factors for recurrence beyond 5 years have not been definitely determined. Therefore, to define the risk factors of late recurrence of RCC, we evaluated the clinical and pathologic factors of patients who had recurrence of RCC beyond 5 years after nephrectomy and patients who had no recurrence beyond 5 years after nephrectomy.
Materials and Methods1. Patient selectionApproval was obtained from the institutional review board at each institution before searching the medical records of patients with RCC. Clinical and pathologic data were collected from four different institutions in Korea. Medical records of 753 patients who underwent radical or partial nephrectomy for RCC between January 2000 and June 2008 were reviewed retrospectively. We excluded patients who were diagnosed with advanced RCC (≥ pT3), did not have follow-up or whose follow-up period was less than 60 months, and those who had relapse within 5 years after nephrectomy. Finally, 225 patients who were treated successfully and had a minimal recurrence-free survival of 60 months were enrolled in the current study. Patient age at the time of surgery, gender, body mass index (BMI), symptoms, creatinine level at diagnosis, tumor size, and pathology were investigated. Pathologic stage was confirmed in accordance with the 2009 American Joint Committee on Cancer TNM staging system [12]. Because enrolled patients’ specimens were confirmed based on the pathologic criteria established before 2009, all of them were analyzed again by highly experienced uropathologists at each hospital. Histologic evaluation of the tumor was analyzed according to the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) guidelines and Heidelberg classification of renal tumors [13]. Fuhrman’s nuclear grading system was applied for assessment of the differentiation of tumor cells [14]. Lymphatic or vascular invasion was recorded if tumor cells were present within an endotheliumlined space without underlying muscular walls.
2. Follow-up protocol and definition of recurrencePatients were followed according to protocols established at each hospital. Typically, all patients were followed every 3 months for the first year after nephrectomy, every 6 months from the second through the fifth year, and annually thereafter. Whenever patients visited the clinic, medical history, physical examination, routine blood tests, and radiologic evaluation were performed. Bone scans and/or chest computed tomography was performed for patients who were suspicious for recurrence or if indicated. Recurrence of RCC was defined as a mass at the operative site, regional lymph nodes, or distant metastasis including a contralateral renal mass.
3. Statistical analysisPatients who had recurrence beyond 5 years after nephrectomy were defined as the late recurrence group and the remaining patients as the recurrence-free group. Mann-Whitney U test was applied for comparison of the clinical and pathologic factors between the two groups. Multivariate logistic regression analyses were used for evaluation of features associated with late recurrence and Cox proportional hazard model was applied to determine the association of features with late recurrence. Analysis was performed using SPSS statistical software ver. 20 (IBM Co., Armonk, NY). Reported p values are two-sided with statistical significance set at p < 0.05.
Results1. Basic characteristics of patientsPatient characteristics of each group are shown in Table 1. Mean age of enrolled patients was 54.35±11.26 years and 94.7% of enrolled patients underwent radical nephrectomy. The number of male patients was 149 (66.2%). Only 14 of 225 patients had recurrence beyond 5 years after nephrectomy. The mean age of the no recurrence group was 54.10±11.47 years and that of the late recurrence group was 58.07±6.56 years (p=0.196). No difference in gender distribution was observed between the two groups. No significant difference in mean follow-up duration, BMI, and creatinine level was observed between the no recurrence group and the late recurrence group (101.02±38.06 vs. 107.07±38.76, p=0.533; 24.68±3.82 vs. 24.03±3.06, p=0.524; 0.95±0.31 vs. 0.94±0.20, p=0.682). However, tumor size of the no recurrence group was significantly smaller than that of the late recurrence group (4.52±2.69 cm vs. 6.98±3.00 cm, p=0.001).
2. Prediction of late recurrenceIn univariate logistic regression analyses, tumor size (odds ratio [OR], 3.487; 95% confidence interval [CI], 1.092 to 11.137; p=0.043), tumor stage > pT1 (OR, 5.394; 95% CI, 1.775 to 16.393; p=0.004), and tumor necrosis (OR, 5.768; 95% CI, 1.655 to 20.097; p=0.008) showed association with late recurrence. In multivariate logistic regression analysis, pathologic T stage > pT1 (OR, 24.771; 95% CI, 2.124 to 288.962; p=0.010), age older than 60 years (OR, 26.718; 95% CI, 1.363 to 523.795; p=0.030), and Fuhrman grade ≥ 3 at the time of nephrectomy were independent risk factors of late recurrence (Table 2). Based on these results, the probability of recurrence-free survival from nephrectomy could be predicted by the Cox proportional hazard model (Fig. 1A and B). Patients with T2 had a significantly lower recurrence-free survival compared to patients with pathologic T1 (log-rank test p=0.007). Likewise, compared with patients whose age at the time of nephrectomy was younger than 60 years, patients whose age was 60 years or older had a significantly lower recurrencefree survival (log-rank test p=0.039). Characteristics of the 14 patients with late recurrence are shown in Table 3.
DiscussionAs a result of advanced radiologic techniques and widespread use of routine abdominal imaging, the recorded incidence of RCC has increased [5]. The change in the incidence of RCC in Korea is not different from that in Western countries and RCC is now the second most common urologic cancer in Korea [1]. Regardless of the incidence of RCC, standard management of RCC is surgical removal of the mass; however, despite adequate surgical treatment of RCC, development of recurrent disease might occur at any time. In particular, 10%-20% of RCC patients who underwent nephrectomy and had no recurrence within 5 years after surgical treatment experienced recurrence beyond 5 years after nephrectomy [7,15-17]. In the current study, 6.2% of patients who had been cancer-free for more than 5 years after nephrectomy had late recurrence. This proportion of late recurrence is low compared with that of previous studies, possibly because we excluded patients with ≥ pT3 or with nodal metastasis or M1. When considering the exclusion criteria, the late recurrence rate observed in our study is similar to that of other studies. These results are important because 5-year disease-free survival and 5-year overall survival rate are used as criteria for successful treatment and prognosis of a particular disease, especially cancer [18,19]. Similar to other cancers, the recommendation for follow-up of clinically localized RCC is that the tests should be performed for 5 years after nephrectomy. Beyond 5 years, guidelines recommend development of the follow-up protocol on a case-by-case basis [20]. However, considering that relapse occurs in approximately 10%-20% of RCC patients, many clinicians have concerns over discontinuing follow-up for a patient who had no recurrence in the first 5 years after nephrectomy. Therefore, many patients who underwent a nephrectomy more than 5 years ago and did not have any relapse are receiving an annual check-up. We believe that our study will be helpful in reducing the concerns of clinicians and in establishing criteria for long-term follow-up.
In the current study, we retrieved data from four different institutions and assessed the effect of clinicopathologic features that might be associated with development of late recurrence in RCC patients. To the best of our knowledge, this is the first effort to establish long-term follow-up criteria for RCC in Korea. We found that patients with late recurrence had larger tumors than patients with no recurrence beyond 5 years after nephrectomy. We also found a significant association of pathologic T stage > pT1, age older than 60 years, and Fuhrman grade ≥ 3 at the time of nephrectomy with late recurrence. The cut-off tumor size for long-term follow-up was determined using a receiver operating characteristic curve. Our results established criteria for long-term follow-up among patients with localized RCC and indicated that patients aged older than 60 years at the time of nephrectomy, tumors of Fuhrman grade ≥ 3, and tumor size larger than 4.5 cm should be followed up closely.
The risk factors of late recurrence have not been not definitely established. Although several studies have addressed this issue, the results were inconsistent. Park et al. [21] reported that age and serum high-sensitivity C-reactive protein levels at the time of surgery might be independent predictive factors for late recurrence of RCC. Ha et al. [22] investigated T1 RCC patients based on multicenter data and found that symptoms at diagnosis or stage T1b tumors were predictive factors for late recurrence. Brookman-May et al. [16] suggested that lymphovascular invasion, Fuhrman nuclear grade 3 or 4, and pathologic tumor stage > pT1 at primary diagnosis are predictive factors for late recurrence beyond 5 years after nephrectomy for RCC. In addition, they developed the PRELANE score system, a risk model that can stratify patients in accordance with their risk for development of delayed recurrence after the operation and has been suggested as a criterion for individualized long-term follow-up. On the other hand, some study groups have reported opposite findings. Uchida et al. [23] did not find any clinical or pathologic features at the time of the initial surgery that predicted delayed recurrence. Adamy et al. [24] recently reported that patients with late recurrence tended to have fewer symptoms, smaller tumors, and less aggressive disease compared to patients with early recurrence; however, because of the small sample size they did not evaluate factors that might be associated with late recurrence.
Like many other studies, the current study had a retrospective design, thus, it has some limitations. First, because it was a multicenter based study, patients enrolled in the study underwent nephrectomy performed by different surgeons. Therefore, surgical skills were not equal, which may result in different outcome. Second, although a similar follow-up protocol was used for all patients within each institution, there may be possible differences in the follow-up patterns among the four institutions, and there might be some differences in the guidelines for radiologists and pathologists of each hospital when reviewing the images and specimens. Also, the definition of late recurrence is vague. Although many other studies on this topic have defined late recurrence of RCC as disease recurrence beyond 5 years after nephrectomy, some studies characterized late recurrence as disease recurrence at 10 years [23,25]. In addition, the number of patients included in all studies is relatively small; therefore, it is difficult to generalize to RCC patients in Korea. Confirmation of our criteria with a standardized protocol and a larger population will be necessary in the future.
ConclusionThe incidence of disease recurrence beyond 5 years after adequately performed nephrectomy among RCC patients with pT1 and pT2 disease was 6.2%. Age ≥ 60 years at diagnosis, pathologic stage > pT1, and Fuhrman grade 3 or 4 are thought to be independent risk factors of late recurrence. We propose that patients with these risk factors should be followed up for the rest of their lives.
Fig. 1.Recurrence-free survival from time of nephrectomy by tumor stage (A) and patient age at nephrectomy (B). 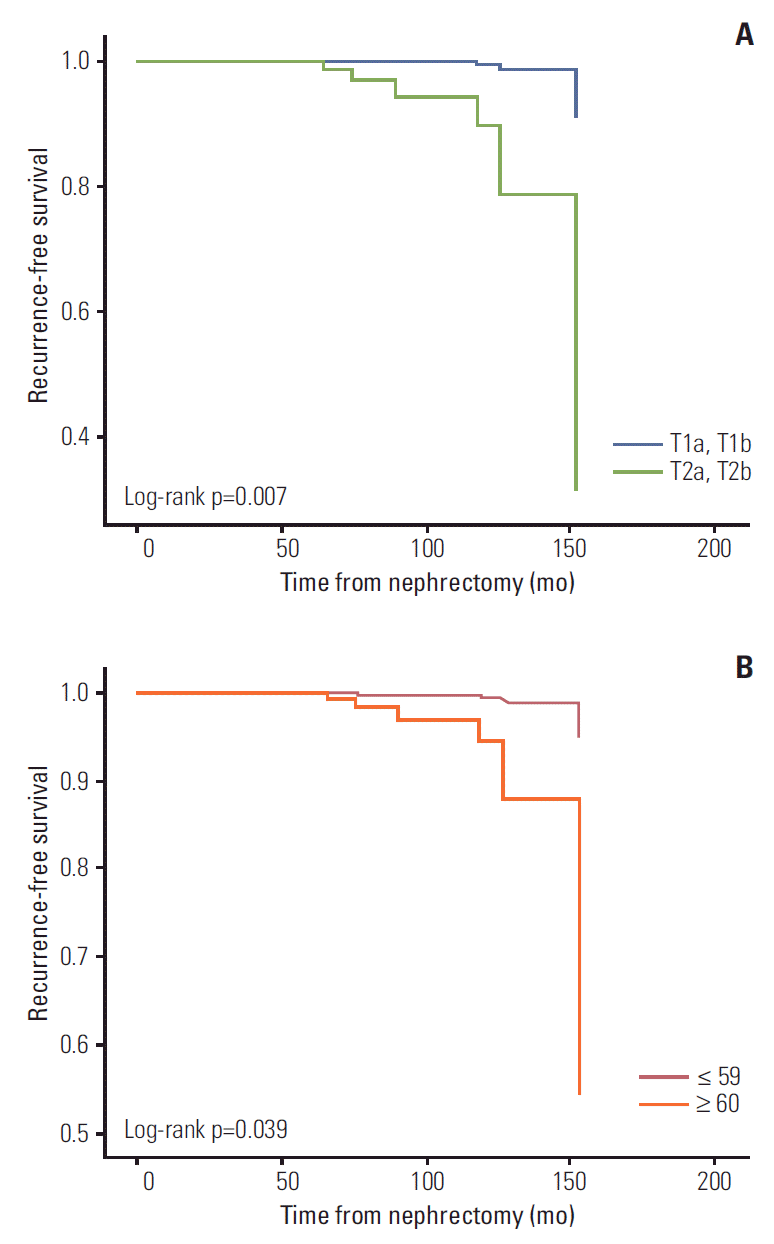
Table 1.Clinical characteristics of the patients Table 2.Univariate and multivariate analysis of factors associated with late recurrence after surgery Table 3.Characteristics of 14 patients with late recurrence References1. Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14.
2. Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406.
3. Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH, et al. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182:1271–9.
4. Motzer RJ, Agarwal N, Beard C, Bolger GB, Boston B, Carducci MA, et al. NCCN clinical practice guidelines in oncology: kidney cancer. J Natl Compr Canc Netw. 2009;7:618–30.
5. Volpe A, Panzarella T, Rendon RA, Haider MA, Kondylis FI, Jewett MA. The natural history of incidentally detected small renal masses. Cancer. 2004;100:738–45.
6. Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Five-year survival after surgical treatment for kidney cancer: a population-based competing risk analysis. Cancer. 2007;109:1763–8.
7. Ljungberg B, Alamdari FI, Rasmuson T, Roos G. Follow-up guidelines for nonmetastatic renal cell carcinoma based on the occurrence of metastases after radical nephrectomy. BJU Int. 1999;84:405–11.
8. Eggener SE, Yossepowitch O, Kundu S, Motzer RJ, Russo P. Risk score and metastasectomy independently impact prognosis of patients with recurrent renal cell carcinoma. J Urol. 2008;180:873–8.
9. Kattan MW, Reuter V, Motzer RJ, Katz J, Russo P. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166:63–7.
10. Leibovich BC, Blute ML, Cheville JC, Lohse CM, Frank I, Kwon ED, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;97:1663–71.
11. Lam JS, Shvarts O, Leppert JT, Pantuck AJ, Figlin RA, Belldegrun AS. Postoperative surveillance protocol for patients with localized and locally advanced renal cell carcinoma based on a validated prognostic nomogram and risk group stratification system. J Urol. 2005;174:466–72.
12. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
13. Kovacs G, Akhtar M, Beckwith BJ, Bugert P, Cooper CS, Delahunt B, et al. The Heidelberg classification of renal cell tumours. J Pathol. 1997;183:131–3.
14. Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63.
15. Nakano E, Fujioka H, Matsuda M, Osafune M, Takaha M, Sonoda T. Late recurrence of renal cell carcinoma after nephrectomy. Eur Urol. 1984;10:347–9.
16. Brookman-May S, May M, Shariat SF, Xylinas E, Stief C, Zigeuner R, et al. Features associated with recurrence beyond 5 years after nephrectomy and nephron-sparing surgery for renal cell carcinoma: development and internal validation of a risk model (PRELANE score) to predict late recurrence based on a large multicenter database (CORONA/SATURN Project). Eur Urol. 2013;64:472–7.
17. Miyao N, Naito S, Ozono S, Shinohara N, Masumori N, Igarashi T, et al. Late recurrence of renal cell carcinoma: retrospective and collaborative study of the Japanese Society of Renal Cancer. Urology. 2011;77:379–84.
18. Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8:541–52.
19. Alanee S, Shukla A. Paediatric testicular cancer: an updated review of incidence and conditional survival from the Surveillance, Epidemiology and End Results database. BJU Int. 2009;104:1280–3.
20. Donat SM, Diaz M, Bishoff JT, Coleman JA, Dahm P, Derweesh IH, et al. Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol. 2013;190:407–16.
21. Park YH, Baik KD, Lee YJ, Ku JH, Kim HH, Kwak C. Late recurrence of renal cell carcinoma >5 years after surgery: clinicopathological characteristics and prognosis. BJU Int. 2012;110:E553–8.
22. Ha YS, Park YH, Kang SH, Hong SH, Hwang TK, Byun SS, et al. Predictive factors for late recurrence in patients with stage T1 clear cell renal cell carcinoma: a multiinstitutional study. Clin Genitourin Cancer. 2013;11:51–5.
23. Uchida K, Miyao N, Masumori N, Takahashi A, Oda T, Yanase M, et al. Recurrence of renal cell carcinoma more than 5 years after nephrectomy. Int J Urol. 2002;9:19–23.
|
|
||||||||||||||||||||||||||||||||||||||||||||