AbstractPurposeThe ability to accurately predict the likelihood of achieving breast conservation surgery (BCS) after neoadjuvant chemotherapy (NCT) is important in deciding whether NCT or surgery should be the first-line treatment in patients with operable breast cancers.
Materials and MethodsWe reviewed the data of 513 women, who had stage II or III breast cancer and received NCT and surgery from a single institution. The ability of various clinicopathologic factors to predict the achievement of BCS and tumor size reduction to ≤ 3 cm was assessed. Nomograms were built and validated in an independent cohort.
ResultsBCS was performed in 50.1% of patients, with 42.2% of tumors reduced to ≤ 3 cm after NCT. A multivariate logistic regression analysis showed that smaller initial tumor size, longer distance between the lesion and the nipple, absence of suspicious calcifications on mammography, and a single tumor were associated with BCS rather than mastectomy (p < 0.05). Negative estrogen receptor, smaller initial tumor size, higher Ki-67 level, and absence of in situ component were associated with residual tumor size ≤ 3 cm (p < 0.05). Two nomograms were developed using these factors. The areas under the receiver operating characteristic curves for nomograms predicting BCS and residual tumor ≤ 3 cm were 0.800 and 0.777, respectively. The calibration plots showed good agreement between the predicted and actual probabilities.
IntroductionNeoadjuvant chemotherapy (NCT) has emerged as the preferred initial treatment for patients diagnosed with locally advanced breast cancer. NCT is administered to those with locally advanced breast cancer to make tumors more operable, to improve outcomes, and to predict treatment outcomes based on patient responses [1]. The indications for NCT have expanded to include patients with early breast cancer and prospective trials showing no survival disadvantage associated with the use of NCT [2,3]. The rate of the overall response to NCT is high, which allows more patients to undergo breast conservation surgery (BCS) with better cosmetic outcomes [3,4]. Furthermore, BCS after NCT does not increase ipsilateral breast tumor recurrence [5].
In a significant number of patients, however, NCT does not result in sufficient tumor reduction to allow BCS. Moreover, a small number of patients experience disease progression during NCT. The relative ineffectiveness of NCT in these patients may be associated with poor chemosensitivity of the tumor and to their clinicopathologic characteristics. Accurate prediction of each patient’s likelihood of achieving breast conservation after NCT is important in establishing a treatment plan for patients with operable breast cancers. Although many models and nomograms have been developed to predict the response of NCT, these models were designed to predict the ability of NCT in achieving a pathologic complete response (pCR) and not the ability of NCT to achieve BCS [6-8]. We hypothesized that the efficacy of the current models could be improved by including clinical, imaging, and pathologic factors. We, therefore, formulated models that increased the accuracy in predicting the likelihood of achieving successful breast conservation and residual tumor size ≤ 3 cm in patients who receive NCT.
Materials and Methods1. Study populationWe reviewed the database of the Seoul National University Hospital Breast Care Center (SNUHBCC) to identify patients with stage II/III breast cancer who received NCT followed by surgery from January 2001 to December 2010. Patients with inflammatory breast cancer, distant metastases at the time of diagnosis, and bilateral breast cancer were excluded. The patient cohort consisted of 513 patients.
All patients were treated preoperatively with ‘anthracycline- and/or taxane-based regimen’ chemotherapy (AC 6.2%, AC-T 2.5%, cyclophosphamide doxorubicin fluorouracil 1.8%, docetaxel doxorubicin 86.5%, paclitaxel gemcitabine trastuzumab 2.9%). Tumor size before treatment and the distance between the nipple and the lesion were determined by breast magnetic resonance imaging (MRI). Patients were considered estrogen receptor (ER)– or progesterone receptor–positive if more than 10% of the cells were stained as positive by immunohistochemistry (IHC). Human epidermal growth factor receptor 2 (HER2) positivity was defined as either HER2 gene amplification by fluorescent in situ hybridization or scored as 3+ by IHC. Suspicious microcalcifications (BIRADS categorized into over C4a) in the area of the lesion on mammography were considered positive for calcification. Breast volume was calculated as described [9]. Clinical and pathologic stages were assessed in accordance to the 7th edition of the American Joint Committee on Cancer (AJCC) cancer staging system.
We considered negative margin when there was no invasive ductal carcinoma and ductal carcinoma in situ (DCIS) at the inked margin microscopically. BCS was considered successful if the margin status was negative at the final surgery. Nipple sacrifice was not considered as breast conservation and central lumpectomy cases were excluded for classifying the surgery type.
2. Statistical analysis and validationA multivariate logistic regression analysis was used to test the associations between clinicopathologic characteristics (including patient age, initial tumor size, ER status, HER2 overexpression, Ki-67 level, p53 expression, breast volume, multifocality, multicentricity, distance from the nipple, presence of calcification, DCIS component, size difference between MRI and sonography, and histologic type) and the ability to perform BCS and residual tumor size ≤ 3 cm after NCT. Backward variable selection was performed to determine the independent covariates. These models were used to develop nomograms, which were built in a training cohort and validated in an independent validation cohort.
The model performance was tested with respect to discrimination and calibration. Discrimination was quantified with the concordance index (c-index), which is identical to the area under the receiver operating characteristics (ROC) curve. Calibration was estimated by graphic representations of the relationships between observed outcome frequencies and predicted probabilities (calibration curves) for the groups of patients defined by quartiles (each quartile included at least 35 patients). The overlap with reference line means perfect agreement on the model. We also compared the performance of our nomograms with those of the M.D. Anderson Cancer Center (MDACC) and the Institute Gustave Roussy (IGR).
All statistical analyses were performed using SPSS ver. 19.0 software (SPSS Inc., Chicago, IL) and R software ver. 2.10.1 (http://www.r-project.org/). All p-values were two sided, and p < 0.05 was considered significant.
ResultsThe baseline characteristics of 513 patients are shown in Table 1. The median age was 42.4 years (range, 24 to 67 years), and 54 patients (10.5%) achieved pCR in response to NCT. NCT consisted of a mean 4.8 cycles of taxane and anthracycline-based preoperative chemotherapy. Thirtyseven out of 513 patients (7.21%) were observed to have a positive resection margin initially. BCS after NCT was performed in 257 patients (50.1%).
1. Nomogram predicting BCS after NCT
Tables 2 shows the results of multivariate logistic regression analyses (Appendix 1). Smaller initial tumor size (p < 0.001), longer distance between the lesion and the nipple (p < 0.001), absence of suspicious calcifications on mammography (p=0.013), and absence of multicentric tumor (p=0.015) were independently associated with BCS rather than mastectomy. A nomogram was developed based on the results of this multivariate analysis (Fig. 1).
2. Nomogram predicting residual tumor size ≤ 3 cmTumor size after NCT is an important and objective factor for considering breast conservation. In this cohort, the mastectomy rate in patients with tumors > 3 cm was 72.7%; whereas the BCS rate in patients with tumors ≤ 3 cm was 63.2% (p < 0.001). Before developing a predictive model, we excluded 94 patients with initial radiologic tumor size ≤ 3 cm. We analyzed the remaining 419 patients to determine the probability of residual tumor size ≤ 3 cm after NCT. Tables 3 shows the results from a multivariate logistic regression analysis (Appendix 2). Multivariate analysis showed that smaller initial tumor size (p < 0.001), absence of DCIS component (p < 0.001), negative ER status (p=0.0023), and higher Ki-67 level (> 15%) (p=0.0148) were independently associated with residual tumor size ≤ 3 cm. A nomogram was developed based on the results of this multivariate analysis (Fig. 2).
3. Validation of the nomogramsOur nomograms predicting BCS and residual tumor size ≤ 3 cm were validated in an independent cohort of 149 breast cancer patients who underwent NCT. Patients were grouped into quartiles of predicted probabilities. To calibrate the nomogram, the actual proportions of patients who achieved BCS and residual tumor size ≤ 3 cm were calculated for each quartile. In the validation group, the nomograms resulted in calibration plots, showing good agreement between the predicted and observed outcomes in most areas (Appendix 3). The areas under the ROC curves of the multivariate model predicting BCS and residual tumor size ≤ 3 cm were 0.800 (95% confidence interval [CI], 0.769 to 0.844) and 0.777 (95% CI, 0.689 to 0.865), respectively. The calibration plots showed a relatively good agreement between the predicted and actual probabilities; however, the predictive probabilities of the third and fourth groups were a little higher and a little lower, respectively, than the actual probabilities of those groups (Appendix 4).
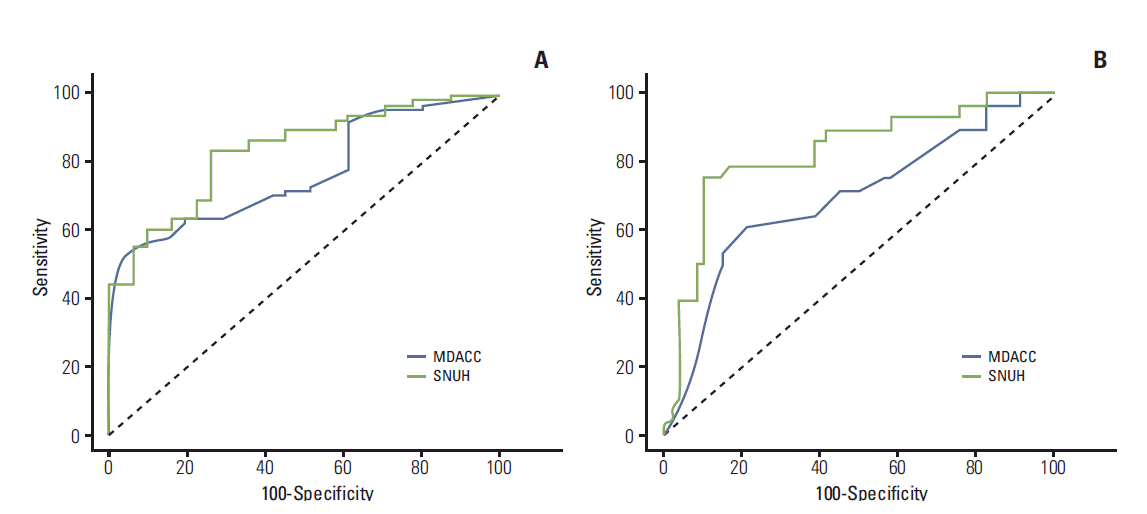
In comparing our nomograms with the previously developed MDACC nomograms, we found that our nomograms showed better performance in predicting BCS and tumor size ≤ 3 cm (Appendix 5). However, it should be noted that we do not have information on the histologic grade of the core needle biopsy specimens.
DiscussionWe have described here the development of nomograms predicting BCS and residual tumor size ≤ 3 cm in breast cancer patients receiving NCT. After matching each factor to the appropriate point, the probability which responds to the total points is figured out. These models were validated in an independent patient set, and showed good performance in terms of discrimination and calibration, with some new factors predicting surgery and tumor response.
These nomograms provided probability estimates that may be useful for individual patients, predicting tumor response to NCT and BCS after NCT. Neoadjuvant therapy is administered to increase the likelihood of performing successful BCS on patients who are not or are borderline eligible for BCS prior to NCT. Our finding, in which 50.1% of patients underwent BCS after NCT, was in agreement with the rates of 38%-68% reported in earlier studies [2,10], suggesting that predictive tools are necessary to assess the likelihood of BCS for patients treated with NCT. Furthermore, as management of breast cancer patients become more multidisciplinary, objective models in clinical settings are going to be necessary for both surgeons and oncologists, when predicting treatment outcomes. Prior to the administration of NCT for breast conservation, it is important to determine whether a patient has a low probability of becoming eligible for BCS. These patients can be offered mastectomy, with or without immediate reconstruction. Alternatively, NCT could be offered after a patient is informed about the low probability of achieving BCS and provides informed consent.
The MDACC nomograms were developed to determine the eligibility for BCS and to predict small residual tumor (≤ 3 cm) for patients receiving NCT [11]. Clinicopathologic factors predicting BCS included the following: ER status, tumor size and grade, multicentricity, and histological type; whereas factors predicting tumor size ≤ 3 cm included ER status, initial tumor diameter, histologic grade and type, and number of chemotherapy cycles. These nomograms were validated in an independent test set of 109 patients, with a c-index of 0.71 for predicting BCS eligibility, although decisions regarding mastectomy versus BCS were reported to be influenced more by subjective factors, such as regional and cultural attitudes than by clinical factors [11]. Furthermore, tumor biology may differ between Asian and Western breast cancer patients [12,13]. Therefore, new tools are needed that can predict which patient in clinical practice are eligible for NCT for breast conserving purposes.
We did not include a histologic grade as a predictive marker for NCT, since the histologic grade in the core biopsy tends to underestimate the grade in the surgical specimen, due in part to the underestimation of the mitotic count [14,15]. The agreement between core needle biopsy and surgical biopsy specimens for grade, mitosis, tubules, and pleomorphism was reported to be 61% [14]. In practice, histologic grade of a presurgical biopsy specimen as a determinant for histologic grade of a surgical specimen may be subjective, dependent on individual pathologists, and our pathologist did not report information from needle biopsy specimens.
The predictive factors of our two nomograms differed, which suggest that any decision of the type of surgery should depend on the surgeon's judgment, not only on objective tumor size after NCT. Indeed, the nomogram for residual tumor size ≤ 3 cm includes greater biologic factors, including ER status, Ki-67 level, DCIS component, and initial size, in agreement with previous models [11]. Furthermore, we found that achieving breast conservation and tumor size ≤ 3 cm were independent of the number of cycles of chemotherapy (> 6 cycles vs. ≤ 6 cycles) or regimen of chemotherapy (including taxane or not).
Although we thought that the initial breast volume would be a predictive factor for breast conservation, we discovered that it was not significant. Rather, the ability to conserve the nipple areola complex was more important for surgical decision making, since the distance between the nipple and the lesion was a significant factor for BCS.
Recently, Mathieu et al. [16] reported that breast cancer index, which is a combination of the two-gene ratio HOXB13: IL17BR(H:1) and the five-gene molecular grade index [17,18], was found to be clinically useful in identifying patients who are not candidates for BCS. In addition, they showed it was the only biologic predictor of BCS in their study. In the near future, some genetic factors predicting chemotherapeutic response or these risk stratifying genes will help our decision making in addition to our model combining clinical, pathological, and imaging variables.
This study had several limitations. The study subjects did not receive homogeneous NCT, and most of HER2 positive patients did not received HER2 targeted therapy; thus, chemotherapy may be a confounding factor. We also did not consider whether BCS was possible before NCT, and we did not distinguish between NCT designed to reduce tumor size or preserve the breast. Moreover, the retrospective nature of the study design made it difficult to obtain information concerning those subjective factors.
ConclusionIn conclusion, we have established a new model to predict BCS and residual tumor size ≤ 3 cm after NCT. This model had a better predictive accuracy than the previously similar models, including novel factors that can affect surgical decision making and tumor response. Use of our nomograms can assist in surgical decision making for patients who are candidates for NCT.
AcknowledgmentsWe thank the Medical Research Collaborating Center (MRCC) of Seoul National University Hospital for the excellent assistance in statistical analysis. This work was supported by National Research Foundation of Korea (NRF) Grant funded by the Korean government (2012M3A9B2028834) and by a grant from the National R&D Program for Cancer Control, Ministry for Health and Welfare, Republic of Korea (A110961).
Fig. 2.Nomogram predicting the probability of residual tumor size ≤ 3 cm. ER, estrogen receptor; DCIS, ductal carcinoma in situ; NCT, neoadjuvant chemotherapy. 
Table 1.Baseline characteristics of the 513 patients who received neoadjuvant chemotherapy followed by surgery Table 2.Multivariate logistic regression analysis of clinicopathologic variables predicting the ability to perform breast conservation surgery after neoadjuvant chemotherapy Table 3.Multivariate logistic regression analysis of clinicopathologic variables predicting the ability to achieve residual tumor size ≤ 3 cm after neoadjuvant chemotherapy References1. Specht J, Gralow JR. Neoadjuvant chemotherapy for locally advanced breast cancer. Semin Radiat Oncol. 2009;19:222–8.
2. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–85.
3. Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:188–94.
4. Rouzier R, Mathieu MC, Sideris L, Youmsi E, Rajan R, Garbay JR, et al. Breast-conserving surgery after neoadjuvant anthracycline-based chemotherapy for large breast tumors. Cancer. 2004;101:918–25.
5. Shin HC, Han W, Moon HG, Im SA, Moon WK, Park IA, et al. Breast-conserving surgery after tumor downstaging by neoadjuvant chemotherapy is oncologically safe for stage III breast cancer patients. Ann Surg Oncol. 2013;20:2582–9.
6. Colleoni M, Bagnardi V, Rotmensz N, Viale G, Mastropasqua M, Veronesi P, et al. A nomogram based on the expression of Ki-67, steroid hormone receptors status and number of chemotherapy courses to predict pathological complete remission after preoperative chemotherapy for breast cancer. Eur J Cancer. 2010;46:2216–24.
7. Rouzier R, Pusztai L, Delaloge S, Gonzalez-Angulo AM, Andre F, Hess KR, et al. Nomograms to predict pathologic complete response and metastasis-free survival after preoperative chemotherapy for breast cancer. J Clin Oncol. 2005;23:8331–9.
8. Keam B, Im SA, Park S, Nam BH, Han SW, Oh DY, et al. Nomogram predicting clinical outcomes in breast cancer patients treated with neoadjuvant chemotherapy. J Cancer Res Clin Oncol. 2011;137:1301–8.
9. Katariya RN, Forrest AP, Gravelle IH. Breast volumes in cancer of the breast. Br J Cancer. 1974;29:270–3.
10. Hatzis C, Pusztai L, Valero V, Booser DJ, Esserman L, Lluch A, et al. A genomic predictor of response and survival following taxane-anthracycline chemotherapy for invasive breast cancer. JAMA. 2011;305:1873–81.
11. Rouzier R, Pusztai L, Garbay JR, Delaloge S, Hunt KK, Hortobagyi GN, et al. Development and validation of nomograms for predicting residual tumor size and the probability of successful conservative surgery with neoadjuvant chemotherapy for breast cancer. Cancer. 2006;107:1459–66.
13. Yoo KY, Kang D, Park SK, Kim SU, Kim SU, Shin A, et al. Epidemiology of breast cancer in Korea: occurrence, high-risk groups, and prevention. J Korean Med Sci. 2002;17:1–6.
14. O'Leary R, Hawkins K, Beazley JC, Lansdown MR, Hanby AM. Agreement between preoperative core needle biopsy and postoperative invasive breast cancer histopathology is not dependent on the amount of clinical material obtained. J Clin Pathol. 2004;57:193–5.
15. Usami S, Moriya T, Amari M, Suzuki A, Ishida T, Sasano H, et al. Reliability of prognostic factors in breast carcinoma determined by core needle biopsy. Jpn J Clin Oncol. 2007;37:250–5.
16. Mathieu MC, Mazouni C, Kesty NC, Zhang Y, Scott V, Passeron J, et al. Breast Cancer Index predicts pathological complete response and eligibility for breast conserving surgery in breast cancer patients treated with neoadjuvant chemotherapy. Ann Oncol. 2012;23:2046–52.
AppendicesAppendix 1.Univariate analysis of clinicopathologic variables predicting the ability to perform breast conservation surgery after neoadjuvant chemotherapyAppendix 2.Univariate analysis of clinicopathologic variables predicting the ability to achieve residual tumor size < 3 cm after neoadjuvant chemotherapyAppendix 3.Calibration plot for breast conservation surgery from nomogram. The x-axis shows nomogram predicted probability and the y-axis shows the actual probability of breast conservation surgery. (A) Training set. (B) Validation set.
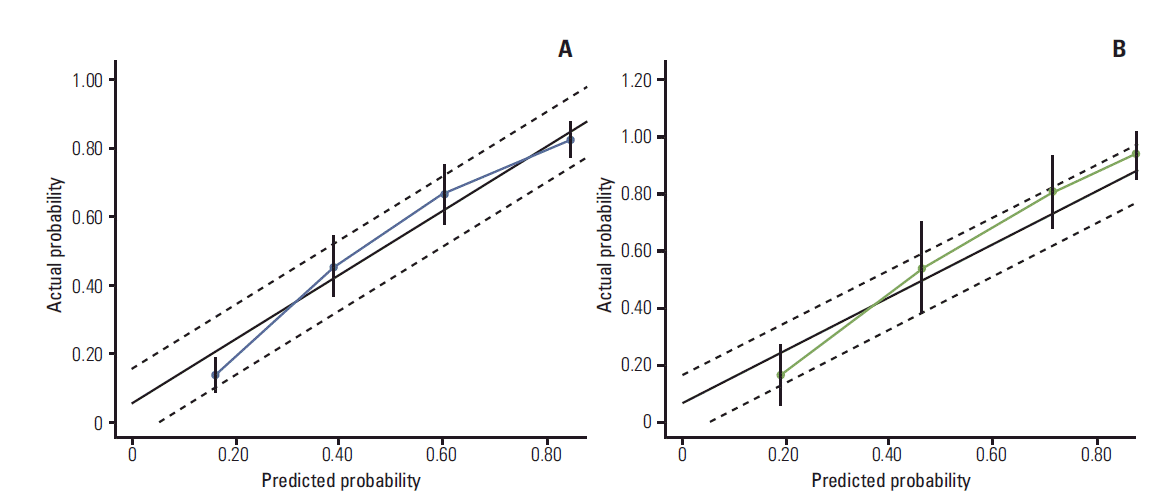
Appendix 4.Calibration plot for predicting residual tumor size ≤ 3 cm from the nomogram. The x-axis shows the nomogram predicted probability and the y-axis shows the actual probability of residual tumor size ≤ 3 cm. (A) Training set. (B) Validation set.

Appendix 5.Receiver operating characteristic curves of Seoul National University Hospital (SNUH) (this study) and M.D. Anderson Cancer Center (MDACC) (Rouzier et al. [11]) nomograms predicting breast conservation surgery (A) and residual tumor size ≤ 3 cm (B).
|
|
|||||||||||||||||||||||||||||||||||||||||