AbstractA 60-year-old woman presented with cerebellar signs including dysarthria and ataxia, after intravenous infusion of cisplatin-based chemotherapy. Several blood tests showed mild neutropenia, normocytic normochromic anemia, but no evidence of a marked hyponatremia. Brain magnetic resonance imaging with diffusion-weighted sequences showed hyper-intense signal abnormalities in the extrapontine region, sparing the basis pontis. Here, we report on the case of a patient with reversible cerebellar ataxia related to extrapontine myelinolysis without hyponatremia after treatment with cisplatin-based chemotherapy for cholangiocarcinoma and discuss the literature on cerebellar ataxia in patients who underwent recent chemotherapy for malignancy.
IntroductionAdams and colleagues initially described central pontine myelinolysis (CPM) as a disease affecting alcoholics and the malnourished [1]. The concept was then extended upon recognition that lesions can occur outside the pons, so-called extrapontine myelinolysis (EPM). CPM and EPM are the same disease, which share the same pathology and time course, but differ in their clinical manifestations [2]. The clinical features of EPM include mental confusion, deterioration of consciousness, extrapyramidal symptoms, ataxia, and dysarthria. Although the mechanism of myelinolysis is not well understood, significant correlation of osmotic balance has been reported in most cases [3]. A recent report questioned the different roles that malignancy might play in myelinolysis, particularly in those without electrolyte imbalance after chemotherapy [4]. We now report on a case of EPM presenting with spontaneously reversible cerebellar ataxia in a patient treated with cisplatin-based chemotherapy, where the blood test showed no prominent fluctuation in the serum sodium level, thus, no correction was necessary.
Case ReportA 60-year-old woman presented to a local hospital after she began to experience right upper quadrant pain, nausea, and vomiting. Abdominal ultrasonography showed marked dilatation of the intrahepatic bile duct (IBD) in the left lobe of the liver and common bile duct with intraductal stones.
She was diagnosed with cholangitis and then referred to our hospital in May 2011 for further investigation and treatment. She underwent laparoscopic left lobectomy for a left IBD stone and choledocholithotomy with t-tube drainage for a common bile duct stone. After one year, follow-up computed tomography (CT) showed no definite abnormalities, but laboratory data showed elevated serum levels of cancer antigen 19-9. Thus, CT scan was performed at three-month interval and a mass was observed at the atrophied right posterior segment of the liver. In addition, a palpable mass was found at the t-tube site and histologic diagnosis of the lesion by excisional biopsy was interpreted as adenocarcinoma (stage IV).
She was referred to our department for palliative chemo-therapy and received treatment with gemcitabine and cisplatin. She neither consumed any alcohol nor smoked and she reported no family history of illness. After eight cycles of gemcitabine and cisplatin (days 1 and 8: cisplatin 25 mg/m2 followed by gemcitabine 1,000 mg/m2; repeated every three weeks), the follow-up CT showed progressive disease, showing an interval increase in the size of the metastatic lesion in the abdomen, thus, the regimen was changed to cisplatin 60 mg/m2 on day 1 and 5-fluorouracil 1,000 mg/m2 on days 1-5.
However, two months after the first infusions of cisplatin and 5-fluorouracil, the patient developed gait disturbances on day 6 of the third infusion. She could not maintain a standing position with her eyes open, and a tandem gait was impossible because of severe positional imbalance. Brain magnetic resonance imaging (MRI) with T2-weighted images (Fig. 1A-C) and diffusion-weighted images (Fig. 1D-F) showed high signal intensity in the extrapontine region, including the corpus callosum, midbrain, internal capsule and bilateral cerebellar peduncles. Laboratory results showed mild neutropenia, normocytic normochromic anemia (hemoglobin 10.8 g/dL), and normal C-reactive protein at 0.08 mg/dL. Her plasma sodium and potassium concentrations were 140 mmol/L and 3.5 mmol/L, respectively. Blood urea nitrogen and creatinine levels were normal. However, the potassium level had decreased to 2.6 mmol/L on day 8. She had been given potassium chloride 600 mg orally until day 3 (days 8-10 of infusion) when selfambulation was possible. Her potassium concentration was altered to 3.2 mmol/L and 3.0 mmol/L on days 9 and 10.
She discontinued cisplatin and only oral doxifluridine (5-fluorouracil) was administered as a salvage therapy. Two months after discharge, prominent clinical improvements were noted and the results of MRI with diffusion-weighted imaging correlated well with clinical outcome (Fig. 2).
DiscussionOsmotic demyelination syndrome is an acute, demyelinating process involving the pons and other locations in the central nervous system. EPM involves widespread regions of the brain, including the midbrain, thalamus, basal nuclei, deep peri-ventricular white matter, and cerebellum. Its manifestations include mental confusion, deterioration of consciousness, irregular respiration, extrapyramidal symptoms, ataxia, and dysarthria [2,5]. Unexpected and initially unexplainable symptoms of patients may thwart efforts at early diagnosis and a following appropriate treatment. Therefore, a diagnosis of EPM should be based on a comprehensive understanding of a patient’s present illness and history, clinical impression and laboratory data, and confirmed by imaging studies, especially MRI. In a recent study, a diffusion-weighted MRI image was reported as a useful tool to facilitate an earlier diagnosis of EPM [6]
In the current case, cerebellar symptoms, especially cerebellar ataxia, were the main clinical findings. Cerebellar ataxia is usually associated with the extension of myelinolysis to the middle or superior cerebellar peduncle [7,8]. The brain MRI of our patient showed high signal intensity in the extrapontine regions, including middle cerebellar peduncle, but sparing the basis pontis shown by diffusion-weighted imaging. Only two cases of cerebellar peduncular myelinolysis without involvement of the central pontine area have been reported; these patients showed an altered state of consciousness and gait disturbance, which were attributable to conditions such as alcoholism or end-stage kidney disease [9,10]. Unlike other cases, in this case, the underlying disease was progressive malignancy.
As with our patient, few cases of cerebellar ataxia in association with malignancy had been reported; this patient with the clinical syndrome of CPM following cisplatin-based chemotherapy for nasopharyngeal carcinoma developed marked slurring of speech and cerebellar ataxia [11]. Another case was reported to show a significant truncal and limb ataxia with bilateral intention tremor in a patient receiving oxaliplatin-based chemotherapy for colon cancer [4].
Our case differed from the first case of cerebellar ataxia in that it was associated with a rapid correction of severe hyponatremia. In many clinical cases, osmotic myelinolysis is most often detected after a rapid correction of serum sodium back to normal levels or a change in serum sodium greater than 25 mmol/L within 48 hours, particularly when associated with hypoxia [2,3]. In the current study, we cannot exclude our patient from having a minor fluctuation in serum sodium levels between the results of blood tests; however, based on the obtained laboratory data, our patient did not develop severe hyponatremia. Therefore, any correction for hyponatremia was unnecessary. In the second case, similar to our patient, this patient manifesting cerebellar ataxia related to CPM showed no evidence of electrolyte abnormalities. However, Dolciotti et al. [4] administered oxaliplatin-based chemotherapy as a treatment for colon cancer. To the best of our knowledge, ours is the first report of a case of spontaneous reversible cerebellar ataxia in EPM without hyponatremia and its correction after cisplatin-based chemotherapy during cancer management.
Regarding electrolyte imbalance, our blood tests performed at the onset of cerebellar ataxia showed hypokalemia. The clinical manifestation showed improvement on day 10, while 600 mg potassium chloride pills were administered for three days (2.6 mmol/L, 3.2 mmol/L, and 3.0 mmol/L on days 8, 9, and 10). A review of published case reports of patients with hypokalemia and a normal serum sodium level [12] found that CPM occurred secondary to hypokalemia, with resolution showing in characteristic MRI findings. In addition, according to a report of two patients with chronic alcohol abuse, a rapid correction of hypokalemia with no disturbance in serum sodium may be associated with pontine swelling and dysfunction, which leads to CPM [13]. However, the low serum potassium level cannot produce a significant shift in effective osmolality as a priori. In addition, in our case, the patient’s gait disturbance was recovered regardless of the maintenance of a low serum potassium concentration after the correction. Thus, hypokalemia and its correction as the cause of cerebellar ataxia related to myelinolysis cannot be completely explained and further study is warranted.
In conclusion, although the etiologies of osmotic demyelination syndrome remain under debate, most clinical studies have characterized this syndrome by gradual neurological symptoms that develop after complete or partial correction of hyponatremia. In the current study, we report on the case of a patient with cerebellar ataxia related to EPM without hyponatremia and its correction after cisplatin-based chemotherapy for cholangiocarcinoma. We hereby suggest more thorough evaluation of the role of malignancy and its related chemotherapy, with conduct of further study for proper management of patients with cerebellar ataxia related to EPM.
Fig. 1.Magnetic resonance imaging of the brain showing a hyperintense lesion of demyelination in the extrapontine areas, including the corpus callosum, midbrain, internal capsule, and middle cerebellar peduncle. No abnormal findings are seen at the basis pontis in magnetic resonance imaging sequences. (A-C) T2-weighted images at axial plane. (D-F) Diffusionweighted images. 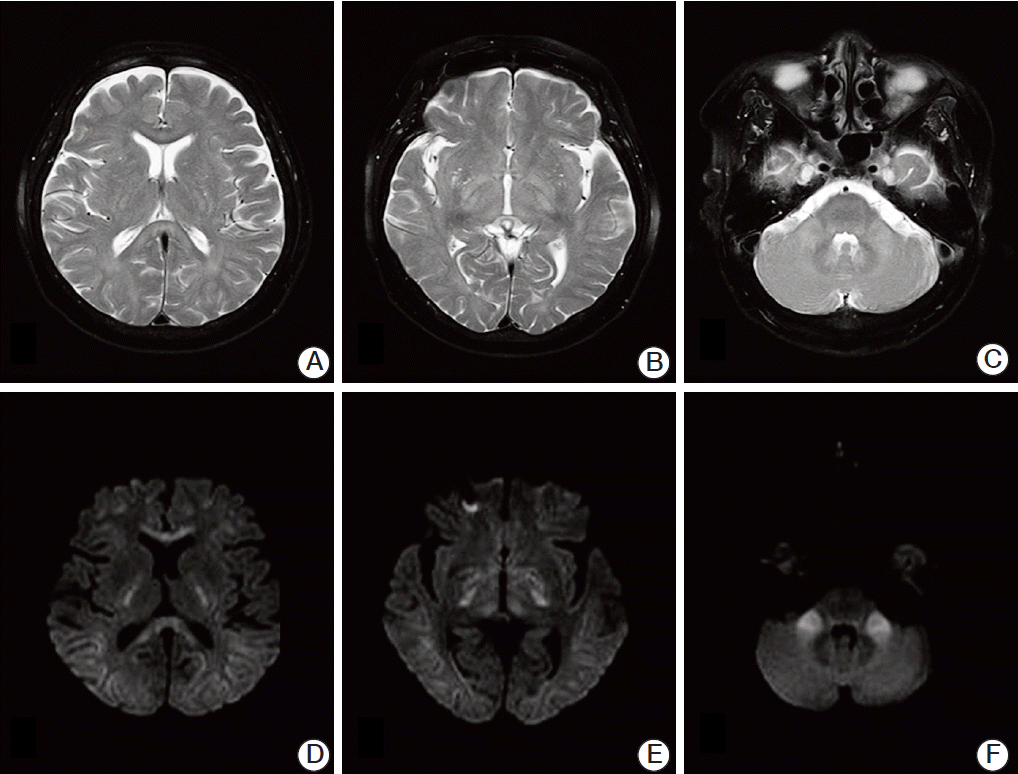
References1. Adams RD, Victor M, Mancall EL. Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. AMA Arch Neurol Psychiatry. 1959;81:154–72.
2. Martin RJ. Central pontine and extrapontine myelinolysis: the osmotic demyelination syndromes. J Neurol Neurosurg Psychiatry. 2004;75 Suppl 3:iii22–8.
4. Dolciotti C, Nuti A, Cipriani G, Borelli P, Baldacci F, Logi C, et al. Cerebellar ataxia with complete clinical recovery and resolution of MRI lesions related to central pontine myelinolysis: case report and literature review. Case Rep Neurol. 2010;2:157–62.
5. Ho VB, Fitz CR, Yoder CC, Geyer CA. Resolving MR features in osmotic myelinolysis (central pontine and extrapontine myelinolysis). AJNR Am J Neuroradiol. 1993;14:163–7.
6. Forster A, Nolte I, Wenz H, Al-Zghloul M, Kerl HU, Brockmann C, et al. Value of diffusion-weighted imaging in central pontine and extrapontine myelinolysis. Neuroradiology. 2013;55:49–56.
7. Steller U, Koschorek F, Strenge H. Cerebellar ataxia with recovery related to central pontine myelinolysis. J Neurol. 1988;235:379–81.
8. Weissman JD, Weissman BM. Pontine myelinolysis and delayed encephalopathy following the rapid correction of acute hyponatremia. Arch Neurol. 1989;46:926–7.
9. Garzon T, Mellibovsky L, Roquer J, Perich X, Diez-Perez A. Ataxic form of central pontine myelinolysis. Lancet Neurol. 2002;1:517–8.
10. Kim J, Song T, Park S, Choi IS. Cerebellar peduncular myelinolysis in a patient receiving hemodialysis. J Neurol Sci. 2007;253:66–8.
11. Yau TK, Yiu HY, Lee WM. Central pontine myelinolysis: report of two occurrences after cisplatin-containing chemotherapy for nasopharyngeal carcinoma. Clin Oncol (R Coll Radiol). 1993;5:395–6.
|
|
|||||||||||||||||||||||||||||||||||||||||||