AbstractPurposeThe purpose of this study is to determine the optimal dose of proton beam therapy (PBT) in hepatocellular carcinoma (HCC) patients.
Materials and MethodsInoperable HCC patients who had naïve, recurrent, or residual tumor to treatment were considered eligible for PBT. Patients received PBT with 60 GyE in 20 fractions (dose level 1; equivalent dose in 2 Gy fractions [EQD2], 65 GyE10); 66 GyE in 22 fractions (dose level 2; EQD2, 71.5 GyE10); or 72 GyE in 24 fractions (dose level 3; EQD2, 78 GyE10). Dose-limiting toxicity was determined by grade ≥ 3 acute toxicity.
ResultsTwenty-seven patients were enrolled; eight, seven, and 12 patients were treated with dose levels 1, 2, and 3, respectively. Overall, treatment was well tolerated, with no dose-limiting toxicities. The complete response (CR) rates of primary tumors after PBT for dose levels 1, 2, and 3 were 62.5% (5/8), 57.1% (4/7), and 100% (12/12), respectively (p=0.039). The 3-and 5-year local progression-free survival (LPFS) rates among 26 patients, excluding one patient who underwent liver transplantation after PBT due to its probable significant effect on disease control, were 79.9% and 63.9%, respectively, and the 3-and 5-year overall survival rates were 56.4% and 42.3%, respectively. The 3-year LPFS rate was significantly higher in patients who achieved CR than in those who did not (90% vs. 40%, p=0.003).
IntroductionHepatocellular carcinoma (HCC) is one of the most commonly diagnosed malignancies and the third leading cause of cancer-related deaths worldwide. HCC is closely associated with hepatitis B and C virus (HBV and HCV) infection, and the majority of patients with HCC have liver cirrhosis (LC), a condition that limits treatment options. Surgical resection and liver transplantation are the mainstays of curative treatment; however, due to the multifocality of HCC development in cirrhotic livers, advanced tumor stage and/or poor hepatic function at diagnosis, and a shortage of graft donors, these methods are restricted to selected patients. Although excellent local control can be achieved with use of percutaneous ablative techniques, including radiofrequency ablation (RFA) and percutaneous ethanol injection therapy, they are restricted to small HCCs and are not suitable for patients with bleeding tendencies, unfavorable anatomic tumor locations, or large tumors [1,2]. Transcatheter arterial chemoembolization (TACE) is clinically useful, particularly for multifocal tumors [3,4]; however, its radical effects are limited histopathologically [5]. Thus, there seems to be a need for effective and less invasive local treatments that can be tolerated by patients with underlying LC and can be used on tumors of any size or location within the liver.
Radiotherapy (RT) techniques using charged particles, including proton beam therapy (PBT) and carbon ion beam therapy, have unique physical properties, the so-called Bragg peak phenomenon, allowing high radiation doses to be delivered to targets within the body while significantly reducing doses to surrounding normal tissues. Previous data of charged particle therapy for HCC patients, using various dose-fractionation schedules, have reported promising results, i.e., good local tumor control and less toxicity [6-15]. However, to the best of our knowledge, the optimal dose of PBT for HCC that results in maximal local tumor control within minimal toxicity has yet to be established. Thus, we conducted this phase I dose-escalation trial to determine the optimal dose of PBT for inoperable HCC patients.
Materials and Methods1. PatientsPatients were recruited to the study based on the following eligibility criteria: 1) pathologically or clinically diagnosed HCC, based on the guidelines of the Korean Liver Cancer Study Group and the National Cancer Center [15]—i) histological confirmation; ii) the presence of risk factors including HBV, HCV, or LC; a serum α-fetoprotein (AFP) level ≥ 400 IU/mL, and an HCC-compatible radiological feature in one or more imaging modalities, i.e., computed tomography (CT), magnetic resonance imaging (MRI), and/or angiography; or iii) the presence of risk factors including HBV, HCV, or LC, a serum AFP < 400 IU/mL, and an HCC-compatible radiological feature in two or more modalities; 2) naïve to treatment, or recurrent or residual tumor after treatment; 3) liver function of Child-Pugh class A or B; 4) Eastern Cooperative Oncology Group (ECOG) performance score of 0 to 1; 5) largest diameter of gross tumor ≤ 10 cm; and 6) adequate function of major organs, including a white blood cell count ≥ 2,000/μL, hemoglobin concentration of ≥ 7.5 g/dL, and platelet count ≥ 25,000/μL. Patients were excluded if 1) the target volume was in direct contact with the gastrointestinal tract; 2) active tumor was present outside the target volume; 3) there was a history of previous RT to the target volume; 4) extrahepatic metastases were detected; and 5) uncontrolled ascites was present. This study was conducted in accordance with the Declaration of Helsinki and all of its amendments. The study was approved by our institutional review board and all patients provided written informed consent before study enrollment. The trial was registered at clinicaltrials.gov (NCT00662246).
2. Pretreatment evaluation and treatment planningAll patients underwent blood tests, including measurements of blood cell counts, liver and renal function tests, titers of HBV and HCV, and AFP. Abdominal dynamic enhanced CT and/or MRI was used to evaluate the extent of HCC. For RT planning, patients were placed in the treatment position (generally, supine with their arms above their head) and immobilized using an arm-up holder to improve setup reproducibility. Contrast enhanced CT images were acquired over 10 respiratory phases, with a 2.5-mm slice thickness, under shallow respiration using a four-dimensional CT simulator (Light-Speed RT, GE Healthcare, Waukesha, WI). All CT images were transferred to a treatment planning system (ver. 8.0, Eclipse, Varian Medical System, Palo Alto, CA), and contours for targets and organs at risk were drawn. The gross tumor volume (GTV) included all detectable tumors, as determined by planning CT images. An internal target volume (ITV) was obtained by summing the GTVs of all respiratory motion phases. The planning target volume (PTV) included the ITV plus a 5-10 mm margin in all directions. An additional 5 mm margin in the craniocaudal direction was included to compensate for uncertainties resulting from respiratory liver motion. Treatment planning was designed to determine the best combination of coplanar and non-coplanar portals to minimize exposure of normal critical organs and the median number of portals was 3 (range, 2 to 4). The primary energy of the proton beams generated from the cyclotron was 250 MeV and all patients were treated with proton of passive double scattering mode (Proteus 235, Ion Beam Application S.A., Louvain-la-Neuve, Belgium). The dose was calculated for the target volume and organs at risk (OAR) and expressed in Gray equivalents [GyE = proton physical dose (in Gray)×relative biologic effectiveness (1.1)]. The treatment was designed so that at least 95% of the PTV would receive 100% of the prescribed dose. The equivalent dose in 2 Gy fractions (EQD2, GyE10), calculated using a linear quadratic model with α/β ratios of 10 for acute effects on tumor and OARs, was used for normal tissue constraints. The maximum dose to the spinal cord could not exceed 45 GyE10; the relative volumes of the total and remaining normal liver that received doses of 30 GyE10 (TLV30 and RNLV30) were below 60% and 50%, respectively [16]; the absolute volumes of the esophagus and stomach that received at least 55 GyE10 were ≤ 2 cm3; and the absolute volumes of the small and large bowel that received at least 50 GyE10 were ≤ 2 cm3.
3. Study designThis phase I dose-escalating study was designed to determine the recommended dose, defined as the minimum of the dose levels not leading to a dose-limiting toxicity (DLT) but leading to the maximum local control, of PBT in patients with HCC. The initial radiation dose was 60 GyE in 20 fractions, increased in 10% increments to 72 GyE in 24 fractions. Dose level 1 consisted of 60 GyE in 20 fractions, 5 fractions/wk; dose level 2 consisted of 66 GyE in 22 fractions, 5 fractions/wk; and dose level 3 consisted of 72 GyE in 24 fractions, 5 fractions/wk. Acute hematologic and non-hematologic toxicities occurring within 90 days from the start of treatment were scored using Common Terminology Criteria for Adverse Events software ver. 3.0 (CTCAE v3.0), with grade 3 or higher toxicity determined to be the DLT. The dose escalation protocol initially consisted of the accrual of six evaluable patients at a given dose level. If no DLT was observed for at least three months, the next set of patients was started at the next dose level. The recommended dose was defined as the highest dose at which the incidence of DLT was less than 33% and the maximum dose was defined as dose level 3, even if none of these patients experienced a DLT, and additional six or more patients were treated at the highest dose level. This study was originally designed for enrollment of six patients with tumor size < 5 cm and six with tumor size 5-10 cm at each dose level; however, enrollment of patients with tumor size 5-10 cm was lower than expected, therefore, our institutional review board approved an amendment, in which at least six patients would be enrolled at each dose level regardless of tumor size (≤ 10 cm).
4. Follow-up and statistical considerationsDuring treatment, acute treatment-related toxicities were assessed weekly in all patients. After completion of PBT, patients were followed up every three months for the first two years and every six months thereafter. Follow-up evaluations consisted of physical examination, a complete blood count, liver-function testing, chest radiography, and abdominal dynamic enhanced CT or MRI. Tumor responses were determined using the modified Response Evaluation Criteria in Solid Tumors criteria (mRECIST) [17]. Although tumors could showed slow regression up to more than one year after PBT [18], the maximum response within one year after PBT was defined as the tumor response due to avoidance of the uncertainties of response evaluation resulting from a high rate of intrahepatic recurrence and effect of salvage treatment. A complete response (CR) was defined as the disappearance of any intratumoral arterial enhancement in all target lesions. A partial response (PR) was defined as a decrease of at least a 30% in the sum of diameters of viable (enhancing) target lesions. Progressive disease (PD) was defined as an increase of at least 20% in the sum of the diameters of viable (enhancing) target lesions. Stable disease was defined as a response that did not qualify as a PR or PD. Recurrence was proven pathologically by surgical resection, biopsy or cytology and/or radiological findings, showing an increase in size over time. Local progression was defined as a regrowth or a new tumor within the treated volume; intrahepatic recurrence was defined as a regrowth or new intrahepatic tumor outside the target volume; and distant metastasis was defined as lymph node recurrence, peritoneal seeding, or metastasis at other extra-abdominal sites. Local progression-free survival (LPFS), disease-free survival (DFS), and overall survival (OS) were defined as the intervals from the commencement of PBT to the date of detection of local progression, any detection of recurrence, and death, respectively.
Fisher exact test and the Kruskal-Wallis test were used to compare the distribution of categorical and continuous variables among the three dose levels, respectively, and Fisher exact test and t-test was used to compare the distribution of categorical and continuous variables between two groups according to tumor response, respectively. Survival rates were calculated using the Kaplan-Meier method and compared using the log-rank test. All statistical analyses were two-sided and were performed using STATA software ver. 9.0 (Stata Corp., College Station, TX). A p < 0.05 indicated statistical significance.
Results2. Tumor response and failure patternsPrimary tumor responses are summarized in Table 2. The CR rates were 62.5% (5/8) for dose level 1, 57.1% (4/7) for dose level 2, and 100% (12/12) for dose level 3 (p=0.039) (Fig. 1). The median time to CR was five months (range, 1 to 10 months) after PBT; however, similar CR rates were observed in patients with primary tumor sizes < 5 cm and ≥ 5 cm (77.3% [17/22] vs. 80% [4/5], p=1.000) (Table 3). At the time of analysis, 18 of the 21 patients (85.7%) who had achieved CR remained locally controlled, with six being disease-free, nine having intrahepatic recurrence, and three having both intrahepatic recurrence and distant metastasis. The three remaining patients (14.3%) who achieved CR showed local failure, including one with intrahepatic recurrence and two with both intrahepatic recurrence and distant metastasis. Of the six patients who did not achieve CR, three (50%) remained locally controlled, with one being diseasefree and two having intrahepatic recurrence; whereas the other three patients showed local failure, including one with intrahepatic recurrence and two with both intrahepatic recurrence and distant metastasis. Of the 27 patients, 20 (74.1%) experienced disease progression at 1.1-44.2 months (median, 7.4 months) after PBT; six (22.2%) experienced local progression at 4.3-44.3 months (median, 11.8 months); 20 (74.1%) experienced intrahepatic recurrence at 1.1-45.3 months (median, 7.4 months); and six (22.2%) experienced distant metastasis at 4.1-36.4 months (median, 18.3 months). Of the 20 patients who had disease progression, 11 received TACE, three received TACE and chemotherapy, two received TACE and RFA with or without PBT, two received TACE and PBT with or without chemotherapy, one received a liver transplantation, and one received no further treatment due to patient refusal. At the time of analysis, 13 patients (48.1%) had died and 14 (51.9%) were alive. The median follow-up period was 31 months (range, 5.2 to 63.4 months) for all patients and 40.4 months (range, 20.7 to 63.4 months) for surviving patients.
Among all patients, the LPFS, DFS, and OS were calculated for 26 patients, excluding one patient who underwent liver transplantation due to its probable significant effect on disease control, and the median OS was 38 months (95% confidence interval [CI], 22.9 to 53 months); the actuarial 3-year LPFS, DFS, and OS rates were 79.9% (95% CI, 64.2% to 95.6%), 17.1% (95% CI, 0% to 36.5%), and 56.4% (95% CI, 36.9% to 75.8%), respectively; and the actuarial 5-year LPFS, DFS, and OS rates were 63.9% (95% CI, 33.1% to 94.7%), 0%, and 42.3% (95% CI, 20% to 64.6%), respectively. The LPFS, DFS, and OS curves according to the three dose levels are depicted in Fig. 2. At dose levels 1, 2, and 3, the 3-year LPFS rates were 71.4%, 83.3%, and 83.3%, respectively; the 3-year DFS rates were 0%, 16.7%, and 20.8%, respectively; and the 3-year OS rates were 25%, 66.7%, and 73.3%, respectively; however, none of these differences was statistically significant (p > 0.05) (Fig. 2A-C). The OS, DFS, and LPFS curves according to tumor response (CR vs. non-CR) are also depicted in Fig. 2. Significantly higher 3-year LPFS (90% vs. 40%, p=0.003) and OS (65.2% vs. 20%, p=0.033) rates were observed in patients who achieved CR, compared with those who did not, whereas DFS rates (20% vs. not reached [NR], p=0.099) did not differ significantly (Fig. 2D-F).
3. ToxicityOverall, treatment was well tolerated with no DLT (Table 4). No significant differences in distribution of acute toxicities were observed among the three dose levels. Within three months after PBT, acute toxicities were transient, easily manageable, and caused no interruption in the treatment course. Of the 27 patients, 22 showed no change in Child-Pugh score, four showed a 1-point decrease and one showed a 1-point increase. A grade 1 late skin and pulmonary reaction was observed in five and four patients, respectively. None of these patients experienced a grade ≥ 2 late toxicity associated with treatment, e.g., mucosal toxicities of the gastro-intestinal tract or radiation-induced liver disease.
DiscussionInoperable HCC lesions remain a therapeutic challenge; however, new modalities of local therapy are emerging. Recently, multicenter studies of charged particle therapy, PBT and carbon ion beam therapy, for inoperable HCC patients have been reported (Table 4) [6-14,21]. Various treatment schedules have been assessed in HCC patients, ranging from 49.5-84 GyE in 4-34 fractions (EQD2, 54.9-102.1 GyE10); these schedules have yielded consistently good clinical outcomes with good tumor control and a relatively low rate of toxicity. The 3- and 5-year LPFS rates have ranged from 75-93% and 81-93%, respectively, with ≥ grade 3 toxicity rates of 0-40%. However, the optimal dose of charged particle therapy, leading to the maximal local tumor control within the minimal toxicity, has yet to be established. Although the current study was a phase I dose-escalation trial that included only 27 patients, with eight, seven, and 12 patients receiving dose levels 1, 2, and 3, respectively, we found that CR rates increased significantly with increasing dose level (62.5%, 57.1%, and 100%, respectively; p=0.039) and that 3-year LPFS rates tended to increase as dose levels increased (71.4%, 83.3%, and 83.3%, respectively; p=0.543) without increasing the rate of DLT. These results suggested that the maximal tolerable EQD2 for optimal local tumor control with minimal toxicity was at least 78 Gy or higher. However, in the current study, the proportion of small tumors (< 5 cm) was slightly higher in dose level 3 (91.7%, 11/12) than in dose level 1 (75%, 6/8) and 2 (71.4%, 5/7). Although the differences were not statistically significant, the tumor size might affect tumor response. Thus, conduct of further large scaled and comprehensive studies should be warranted.
In addition, although previous studies included HCC patients with unfavorable prognostic characteristics (i.e., recurrent tumors, advanced stage, unfavorable tumor location, and high grade of hepatic impairment), their 3- and 5-year OS rates ranged from 45% to 50% and from 22.2% to 38.7%, respectively (Table 5) [6-14]. In particular, Chiba et al. [7] reported that the 5-year OS rate for patients with a solitary tumor and less impaired hepatic function (Child-Pugh class A) was 53.5%. These results suggested that PBT or carbon ion beam therapy may have a major role in treatment of patients with HCC, both those with favorable characteristics (e.g., small, solitary tumors and good liver function) and those who are difficult-to-treat (e.g., large tumors and recurrent tumors after a previous curative treatment). Similarly, although our study population had unfavorable clinical characteristics, including advanced tumor stage (II, 29%; III, 56%; IVA, 15%), recurrent or residual tumors after previous treatment (96.3%), large tumor size (18.5%, ≥ 5 cm), and somewhat impaired hepatic function (Child-Pugh class A, 89%; Child-Pugh class B, 11%), the 5-year OS rate for all patients was 42.3%. In a cohort analysis of 904 patients with HCC treated at our institution [22,23], the 5-year OS rates for patients with stage I-II tumors undergoing surgical resection, RFA, and TACE were 80.1%, 70%, and 52.8%, respectively, and the 5-year OS rates for patients with stage III tumors undergoing surgical resection and TACE were 60.7% and 17.0%, respectively. It is impossible to simply compare these results among the different patient populations studied because the survival of patients with HCC depends largely on both the degree of impairment of hepatic function resulting from co-existing LC and the tumor stage. However, considering disease stage, the results of PBT in the current study are comparable with those of other treatment modalities, suggesting that PBT can be as effective as currently established standard treatments, such as surgical resection, RFA, and TACE.
This study had several limitations. First, because it was a step-wise dose escalation study evaluating a relatively small number of patients (n=27), we could not show positive relationships between long-term outcomes, such as LPFS and OS, and the effects of dose-escalation. Therefore, we compared relative short-term tumor response using mRECIST criteria at the three dose levels. In the current study, relative short-term tumor response using mRECIST criteria does not perfectly coincide with long-term local tumor control: 18 of the 21 patients (85.7%) who had achieved CR remained locally controlled at the time of analysis and three of the six patients (50%) who did not achieve CR remained locally controlled. However, in the current study, one of three patients who remained locally controlled underwent liver transplantation after PBT due to intrahepatic recurrence outside of the RT field. In addition, we found that CR rates increased significantly with increasing dose level (62.5%, 57.1%, and 100% at dose levels 1, 2, and 3, respectively; p=0.039) and that 3-year LPFS rate was significantly higher in patients who achieved CR compared with those who did not (90% vs. 40%, p=0.003). These findings suggest that tumor response using mRECIST criteria could be a useful surrogate marker for long-term tumor control, i.e., LPFS, after treatment. Second, we did not find that dose-escalation of PBT improved the tumor response and subsequently improved OS because the rate of intrahepatic recurrence outside the RT field was high (74.1%), similar to rates in previous studies (35-85%) [6-14,24]. The co-occurrence of high local tumor control rate and high disease progression rate is explained by the multifocal nature of HCC in the cirrhotic liver and the advanced tumor stage in the study population (II, 29%; III, 56%; IVA, 15%). However, previous data [6-14] and the current study have consistently suggested that delivery of sufficiently high doses of RT can potentially control HCCs. Thus, we start a phase II study to confirm the effectiveness and feasibility of PBT for HCC patients with recurrent or residual disease (http://www.clinicaltrials.gov; NCT01643824).
ConclusionIn conclusion, although a maximum tolerance dose of PBT was NR and additional larger and more comprehensive studies are needed to determine the most appropriate dose fractionation schedule for optimal local tumor control with minimal toxicity, our results suggest that at least 78 GyE10 of EQD2 is needed to achieve sufficient local tumor control in patients with inoperable HCC.
AcknowledgmentsThis study was supported by a National Cancer Center Grant (NCC 1310080, 1340940 and 1241110).
Fig. 1.Complete response of a primary tumor to proton beam therapy (PBT). (A) Pretreatment computed tomography (CT) scan showed the primary tumor (arrow). (B) The patient underwent PBT. (C) CT scan three months after PBT showed complete remission of the primary tumor (arrow). 
Fig. 2.Local progression-free survival (LPFS) (A and D), disease-free survival (DFS) (B and E), and overall survival (OS) (C and F) curves relative to the three radiation dose levels (DLs) and tumor response (complete response [CR] vs. non-CR). CI, confidence interval; NR, not reached. a)Log-rank test. 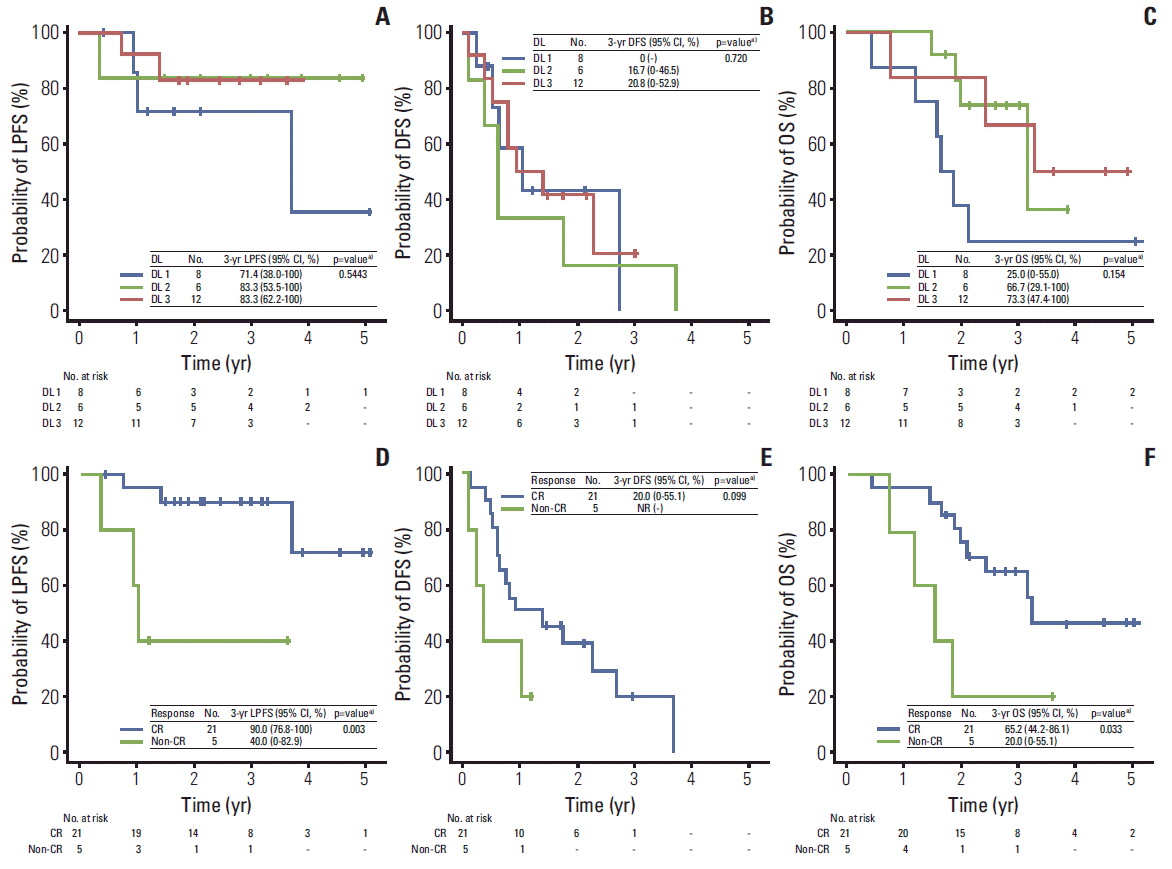
Table 1.Patient characteristics
ECOG PS, Eastern Cooperative Oncology Group performance status; LC, liver cirrhosis; HBV, hepatitis B virus; HCV, hepatitis C virus; CPC, Child-Pugh classification; Dx, diagnosis; PBT, proton beam therapy; MELD, model for end stage liver disease; AFP, α-fetoprotein; mUICC stage, modified International Union Against Cancer stage [19]; BCLC stage, Barcelona Clinic Liver Cancer stage [20]; TACE, transcatheter arterial chemoembolization; RFA, radiofrequency ablation; PEIT, percutaneous ethanol injection therapy; OP, operation. Table 2.Tumor response relative to the three radiation dose levels
Table 3.Comparison of patient characteristics according to tumor response
CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; HBV, hepatitis B virus; HCV, hepatitis C virus; CPC, Child-Pugh classification; Dx, diagnosis; PBT, proton beam therapy; MELD, model for end stage liver disease; AFP, α-fetoprotein; mUICC stage, modified International Union Against Cancer stage; BCLC stage, Barcelona Clinic Liver Cancer stage. Table 4.Toxicity among patients treated with the three radiation dose levels
Table 5.Published results on clinical outcomes of charged-particle therapy in hepatocellular carcinoma patients
mUICC stage, modified International Union Against Cancer stage; Dose/Fx scheme, Dose/Fractionation scheme; EQD2, equivalent dose in 2 Gy fractions, using a linear quadratic model with α/β ratios of 10 for tumor; LPFS, local progression-free survival; OS, overall survival; Rib Fx, rib fracture; RP, radiation pneumonitis; GIT, gastrointestinal toxicity; HT, hematologic toxicity; PH group, porta hepatis group; Non-PH group, non-porta hepatis group; LT, liver toxicity. References1. Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–30.
2. Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714–23.
3. Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–71.
4. Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–9.
5. Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization: a histopathologic study of 84 resected cases. Cancer. 1994;73:2259–67.
6. Bush DA, Hillebrand DJ, Slater JM, Slater JD. High-dose proton beam radiotherapy of hepatocellular carcinoma: preliminary results of a phase II trial. Gastroenterology. 2004;127(5 Suppl 1):S189–93.
7. Chiba T, Tokuuye K, Matsuzaki Y, Sugahara S, Chuganji Y, Kagei K, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11:3799–805.
8. Fukumitsu N, Sugahara S, Nakayama H, Fukuda K, Mizumoto M, Abei M, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2009;74:831–6.
9. Imada H, Kato H, Yasuda S, Yamada S, Yanagi T, Kishimoto R, et al. Comparison of efficacy and toxicity of short-course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother Oncol. 2010;96:231–5.
10. Kato H, Tsujii H, Miyamoto T, Mizoe JE, Kamada T, Tsuji H, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59:1468–76.
11. Kawashima M, Furuse J, Nishio T, Konishi M, Ishii H, Kinoshita T, et al. Phase II study of radiotherapy employing proton beam for hepatocellular carcinoma. J Clin Oncol. 2005;23:1839–46.
12. Komatsu S, Fukumoto T, Demizu Y, Miyawaki D, Terashima K, Sasaki R, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 2011;117:4890–904.
13. Mizumoto M, Tokuuye K, Sugahara S, Nakayama H, Fukumitsu N, Ohara K, et al. Proton beam therapy for hepatocellular carcinoma adjacent to the porta hepatis. Int J Radiat Oncol Biol Phys. 2008;71:462–7.
14. Nakayama H, Sugahara S, Fukuda K, Abei M, Shoda J, Sakurai H, et al. Proton beam therapy for hepatocellular carcinoma located adjacent to the alimentary tract. Int J Radiat Oncol Biol Phys. 2011;80:992–5.
15. Park JW; Korean Liver Cancer Study Group and National Cancer Center. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol. 2004;10:88–98.
16. Kim TH, Kim DY, Park JW, Kim SH, Choi JI, Kim HB, et al. Dose-volumetric parameters predicting radiation-induced hepatic toxicity in unresectable hepatocellular carcinoma patients treated with three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys. 2007;67:225–31.
17. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60.
18. Ohara K, Okumura T, Tsuji H, Min M, Tatsuzaki H, Chiba T, et al. Clearance of parenchymal tumors following radiotherapy: analysis of hepatocellular carcinomas treated by proton beams. Radiother Oncol. 1996;41:233–6.
19. Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatol Res. 2002;24:395–403.
20. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–38.
21. Kawashima M, Kohno R, Nakachi K, Nishio T, Mitsunaga S, Ikeda M, et al. Dose-volume histogram analysis of the safety of proton beam therapy for unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;79:1479–86.
22. Park KW, Park JW, Choi JI, Kim TH, Kim SH, Park HS, et al. Survival analysis of 904 patients with hepatocellular carcinoma in a hepatitis B virus-endemic area. J Gastroenterol Hepatol. 2008;23:467–73.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||