INTRODUCTION
Nephron-sparing surgery (NSS) is gaining popularity as a treatment for small renal masses that are suspected to be malignant. This surgery has shown similar results compared to that of radical nephrectomy for the long-term survival and local tumor recurrence (1). Open partial nephrectomy (OPN) has been the reference standard for NSS, and laparoscopic partial nephrectomy (LPN) has also shown excellent surgical results and an ability to control cancer when it is used to treat small peripheral tumors (2). Although LPN has the advantages of combining minimal invasiveness and preservation of the renal function, more advanced technical dexterity is required from the surgeon, the complication rate is higher and a longer warm ischemic time is needed compared to OPN, and these factors limit the role of LPN and especially for complicated cases (3). These are the reason why OPN is currently still the standard NSS.
Ablative techniques that destroy tumor tissue instead of removing it have gained interest and mainly because of the decreased morbidity, a shorter hospital stay, preservation of the renal function and their ability to treat patients who would otherwise be poor surgical risks (4). Among the alternative ablation techniques, cryoablation is the best documented and studied ablative procedure for treating renal tumor (5). As for the approach to cryoablation, the laparoscopic renal cryoablation (LRC) procedure has distinct advantages over the percutaneous approach, including easy access to anterior or hilar lesions and real time image can be applied by using intraoperative ultrasonography (IOUS).
For the urologist, the main obstacle for selecting LRC as a tool to treat patients with small renal tumors is the lack of long-term oncologic results and the lack of any comparative study with other NSS procedures. Until now, the long-term results of LRC have not been published. A prospective trial that compares standard procedures with LRS is needed to validate the role of this developing modality in the clinical field. Therefore, we present a matched trial comparison of LRC with OPN, which is the established procedure for NSS, for the treatment of small renal cell carcinoma (RCC). We report here on the intermediate term follow up results, and especially the oncologic and surgical outcomes.
MATERIALS AND METHODS
From April 2004 to June 2007, LRC using ultra-thin cryoprobe was performed on 35 patients with renal tumors. We selected the patients who had pathologically confirmed RCC on their needle biopsy and the tumor size was smaller than 4 cm. Finally, 20 of the 35 patients (the LRC group) were prospectively enrolled in this study. These patients were matched with 20 patients (the OPN group) who were selected based on the pre-operative characteristics of the tumor and the patients' characteristics, and these 20 control patients were selected from a pre-existing database of the 72 patients who had undergone OPN during the same period. All the patients who underwent OPN at our institution were registered prospectively in a specific database that included all the important information, such as age, gender and the tumor location, size and pathology, and they were all followed up under a strict guideline that was also applied for the LRC patients. This allowed us to match the parameters of the 20 LRC patients against those of the 20 OPN patients. All the patients underwent preoperative CT or MRI, which revealed a solid mass with an enhancing portion in the kidney, and this was suggestive of a malignancy. For the characteristics of tumor, we defined endophytic tumors as those tumors with less than 40% of the lesion extending off the surface of the kidney, and a hilar tumor was defined as those tumors positioned medially within 5 mm of the renal artery or vein (6). The variables related to the surgical and oncologic outcomes were evaluated and then compared for each group.
In addition to the traditional indications for NSS, including bilateral tumors and the patients with a solitary kidney or renal insufficiency, those patients who electively chose to undergo a NSS procedure were also enrolled. All the treatment options for renal tumor were preoperatively explained and the final treatment decision was made based mainly on the surgeon's and patients' preference after discussing the risks for each procedure and considering the absence of data on the long-term outcomes for cases that undergo LRC. Each LRC and OPN procedure was performed by a single surgeon (SH Kang and DK Yoon, respectively), who used nearly identical techniques. Approval was granted by the local institutional review board before initiating this study, and all the patients provided their informed consent.
All the LRCs were administered under general anesthesia with using the technique briefly described below. The tumors anterior to a horizontal line within the coronal plane through the renal hilum were generally approached transperitoneally, and tumors posterior to this line were approached retroperitoneally. For the cases of endophytic tumors, a 7Fr ureteral indwelling catheter was inserted preoperatively. A standardized technique using three ports was used for the laparoscopic procedures. Real-time IOUS (Aloka Dynaview II, Americanlab, Maimi, FL) was used in all the cases to identify the lesion and to determine the degree that the ice ball extended and covered the tumor. The kidney was mobilized and Gerota's fascia was opened to facilitate identification of the tumor. The fat overlying the tumor was widely removed, and then the tumor was retrieved for pathology examination. Before insertion of a cryoprobe, more than two needle biopsies were taken from the tumor before insertion of a cryoprobe. Depending on the tumor size and its characteristics, single or multiple 1.47 mm cryoprobes (IceRod, Oncura, Plymouth Meeting, PA) were deployed. IOUS confirmed the position of the cryoprobe tip beyond the deep margin of the lesion. Two temperature probes were then inserted into the middle and the peripheral margins of the tumor to assure that a temperature below -40℃ was reached within the tumor, which is usually required to effectively destroy malignant renal tissue (7). In all cases, a double-freeze cycle was applied in all the cases, with an intervening thawing process between the cycles. Each freeze cycle was continued until the ice ball extended 1cm circumferentially beyond the edge of the tumor. After the second thaw allowed safe removal of the cryoprobe, hemostasis was achieved by filling the probe tract with fibrin glue (Baxter, Deerfield, IL) and Surgicel (Johnson & Johnson, Irvin, CA). In OPN, the standard access for kidney exposure was obtained via a retroperitoneal flank incision that was made above the eleventh or twelfth rib. The transperitoneal approach was used for the hilar or medially located tumor that required meticulous pedicle dissection. Temporary renal vascular occlusion was used in all the cases.
The patients were initially evaluated at one month and then they were evaluated every 3 months during the first year, every 6 months during the second year and then annually with a medical history, physical examination, blood pressure measurements, contrast enhanced CT or MRI, chest X-way, serum electrolyte measurements, liver function tests and renal function tests. For the LRC cases, a lack of enhancement on CT or MRI along with a stable or decreased tumor size was considered as successful treatment. Recurrence in the LRC group was defined as an increasing tumor size or the lack of tumor shrinkage, along with enhancement (8). For the OPN cases, the radiologic finding of local recurrence was defined as a mass with strong enhancement at the excision site or at the perinephric space, with an increase in size on the subsequent follow-up image (9).
RESULTS
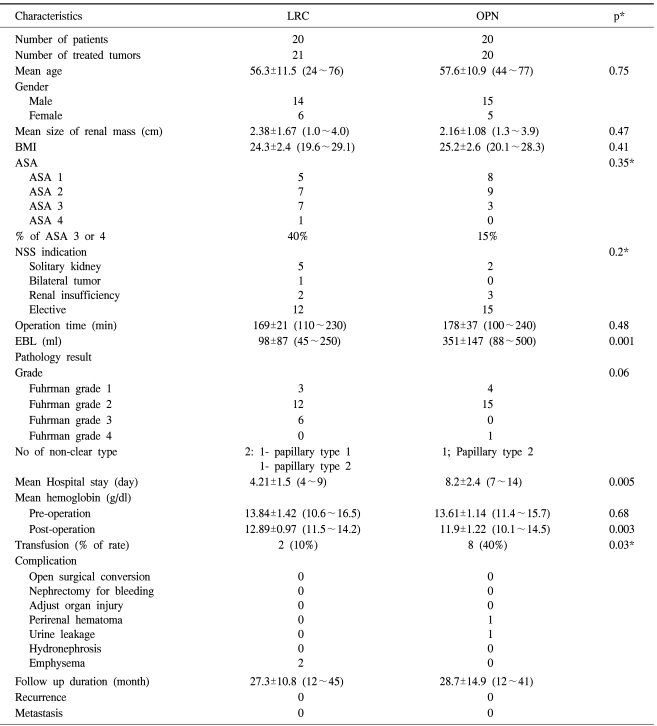
The mean age and mean tumor size were 56.3 years (range: 24~76 years) and 2.38 cm (range: 1.0~4.0 cm) in the LRC group, and 57.6 years (range 44~77 years) and 2.16 cm (range 1.3~3.9 cm) in the OPN group. Due to a bilateral case, 21 tumors were treated in the LRC group. The two groups were similar in age, gender, BMI, the American Society of Anesthesiology (ASA) score and the tumor characteristics (Table 1). For the LRC group, 12 out of 20 patients were selected due to elective indications, and in the OPN group, 15 out of 20 patients were also selected due to elective indications. Non-hilar tumor that was located in the lower pole was the most frequent character of the mass in both groups. An exophytic mass was most common in both group; fifteen patient in the LRC group and sixteen patients in the OPN group had an exophytic mass. For the cases with an endophytic mass, we could successfully identify the tumor margins with the aid of the IOUS.
The mean operation time was similar for both groups. Most tumors in both groups were the conventional type, except for two patients in the LRC group and one patient in the OPN group, who were all confirmed as having the papillary type. The estimated blood loss (EBL), which was reflected by the hemoglobin changes, was less in the LRC group than that in the OPN group (98±87 ml vs 351±147 ml, respectively, p=0.001), and as a consequence, the transfusion rate was higher in the OPN group (10% vs 40%, respectively, p=0.03).
Among the two patients who needed transfusion in the LRC group, one had preoperatively pernicious anemia due to a previous gastrectomy for stomach cancer, and the other had subcutaneous hematoma due to bleeding at the trocar insertion site. The hospital stay was significantly shorter for the LRC group with a mean of 4.21 days (range: 2~7 days) than that for the OPN group (4.21±1.5 days vs 8.2±2.4 days, respectively, p=0.005). Two patients in the LRC group had subcutaneous emphysema, which was treated effectively with conservative measures. But in the OPN group, one patient experienced urine leakage, which was detected by an increased drainage volume and an increased creatinine level. After placing an indwelling ureteral stent for 14 days, the urine leak resolved without sequelae. One patient had a perirenal hematoma, which was identified by the CT scan taken on 3 day after operation and the decreased post-operative hemoglobin level; this hematoma was resolved on the CT scan taken at 14 days after operation. One patient had neuropathic pain that required prolonged pain management for 6 weeks, and this was controlled with oral analgesics. Other than these, there were no major or minor complications.
All of the enrolled patients were followed up for more than 12 months. The mean follow up in the LRC group was 27.26 months (range: 12~45 months), and in OPN group, the mean follow up was 28.66 months (range: 12~41 months). During this period, all the patients in both groups remained disease-free with no evidence of local recurrence or metastases.
DISCUSSION
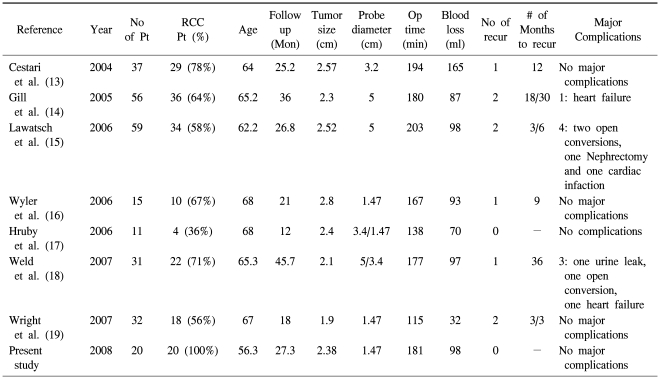
The treatment options for small renal masses have expanded during the past decade. The increased use of cross-sectional image during the same period had led to "stage migration" with most masses being diagnosed at an early stage (10). For these small renal masses, OPN has replaced radical nephrectomy as the treatment of choice. Due to its minimal invasiveness and promising oncologic outcome, LPN has gained interest, but it is also associated with a higher complication rate and a longer learning curve than that for OPN (11). Ablation using a cryoprobe or radiofrequency probes offers the advantages of minimally invasive surgery with a significantly lower complication rate than that for LPN (12). Furthermore, many of these masses are being diagnosed in elderly patients with co-morbidities and who are not good candidates for partial nephrectomy or a major surgical procedure. In situ thermal destruction of renal masses through the creation of lethal cold temperatures offers a safer alternative, in theses circumstances. Besides, many of the early and intermediate term reports have shown promising results for LRC, and the results of these reports are summarized in Table 2 (13-19). The two published LRC series with a minimum of three-year follow-up demonstrated radiographically documented success rates of 97% and 96%, respectively (14,18). However, the lack of sufficient long term data for the procedure's efficacy data has been the most common reason for not offering this ablation technique for treating renal masses, which was shown in a recent survey on the current practice patterns of ablation techniques for 112 academic urologists (20).
The present study provides further evidence of the efficacy of cryoablation for managing small renal masses. All the cases of LRC of this series were confirmed to have RCC. As shown in Table 2, most of the investigators have reported the results of LRC with enrolling patients who were screened by only radiologic evaluation; as a consequence, there is probability of overestimation in interpreting the oncologic efficacy of cryoablation because not all the patients enrolled in those trials were confirmed as having RCC. Furthermore, it is well known that only 78% of resected renal tumors smaller than 4 cm on the preoperative imaging study are malignant, meaning unnecessary operations are done on 22% of the patients (21). Therefore, to reveal the actual oncologic efficacy of cryoablation for controlling RCC, we focused on the patients who were pathologically confirmed as having RCC, and the minimum follow-up was 12 months. In our series, all the patients in the LRC group were recurrence-free and progression-free during the mean follow up of 27 months, and these results were comparable with that of OPN, which is the reference standard for NSS. To acquire a proper specimen for histology, we conducted multiple needle biopsies for all the cases. The most bothersome complication pertaining to use of multiple needle biopsies is bleeding from the biopsy track. To reduce this bleeding, we initiated a freezing cycle immediately on removal of the last biopsy needle. Furthermore, following completion of the second thaw phase, we removed the cryoprobes before the ice slush from the cryoablated lesion could melt so as to maintain a rigid needle track through which we then injected fibrin glue. Through such techniques, significant bleeding or perirenal hematoma requiring surgical conversion or nephrectomy did not occur in the LRC group.
Other complications in the LRC group, besides bleeding from the probe track, were rare and no major complications requiring prolonged hospitalization or invasive treatments occurred. Compared with the LRC group, two patients in the OPN group had urine leakage and one patient had perirenal hematoma. The low incidence of complications when performing LRC might be related to the small diameter of the cryoprobe. Ultra-thin 1.47 mm cyroprobes, which were used in our series, have a relatively low risk of both bleeding and disastrous renal fracture compared to that of the other larger sized cryoprobes (16,19). Surgery related problems such as urine leakage, performing nephrectomy for bleeding and incurring adjacent organ damage have occurred in the studies that used relatively large diameter cryoprobes. In contrast, no major complications have been reported in three previous studies, including our own, that used ultra-thin cryoprobes of 1.47 mm (Table 2) (16,19).
The patient age was noticeably younger in our series (56.3 years) as compared to the recent studies concerned with LRC. Though the current indications for ablative therapy have not been strictly defined, it has usually been agreed upon that the ideal candidates are patients with small renal mass (<4 cm) and those patients with significant comorbidities; the absolute contraindication is uncorrectable bleeding diathesis, and the relative contraindications are cystic masses, a young patient age and tumors larger than 4 cm. While age is certainly an important factor for deciding the proper treatment options to manage small renal masses, it is important to keep in mind that significant variation exists for the patients within the same age groups. In our series, although the difference of ASA score, which reflects the comorbidities of the patients, did not achieve statistical significance, the percentage of patients in the LRC group with an ASA score over 3 was nearly three times as high as that in the OPN group. Therefore, despite the fact that our patient group was significantly younger than that of the other previous studies, the actual application did not greatly differ from the current standards for performing LRC. The recent reports on the 5 years efficacy for LRC indicate that the feasibility of LRC for younger age patients or for larger tumors is comparable to that for OPN, but to determine the adequate indications for performing LRC for younger age patient, a follow up period comparable to that applied to OPN, which we used as a reference standard, should be applied to younger patients who are without sever co-morbidities for making proper comparison.
Another advantage of using ultrathin 1.47 mm cryoprobes is that multiple cryoprobes can be used for small lesions. Except for one case, we used multiple cryoprobes and the mean number of cryoprobes, except thermosensors, needed for the operation was 2.43 (range: 1~4). For reliable tumor destruction and obtaining an adequate margin for the ice ball, the exact positioning of the cryoprobe in the centre and peripheral margin of the tumor is the most important process. The use of multiple cryoprobes might hypothetically increase the efficiency of freezing by extending the coldest isothermal line, compared with using a single probe, and the distribution of the probes across the tumor might also compensate for an asymmetric tumor shape. However, for the case of an endophytic tumor, and especially the tumor that is directly in contact with the collecting system, the use of multiple cryoprobes can induce urine leakage if the collecting system is significantly involved during ablation. But in previous studies, targeted renal pelvic cryoablation resulted in no case of urinary extravasations from 15 total lesions in a swine model (22), and six patients with central tumor who were treated by percutanous cryoablation revealed no clinical evidence of ureteral sequelae (23). Furthermore, most of the previously reported clinical cases of urine leakage could be managed conservatively with a ureteral indwelling catheter (24). In our series, all the endophytic masses in both groups were directly in contact with the collecting system. To decrease inducing adverse events related to the collecting system, a pre-operative ureteral indwelling catheter was inserted for all such cases in the LRC group; the catheters were then removed 7 day after operation. No hydronephrosis or flank pain occurred during follow-up. So, we surmise that it would be better to insert an indwelling catheter preoperatively for the case of an endophytic tumor.
We recognize the limitations of the present study, including the intermediate length of the follow up, the modalities used for follow up and the inherent selection bias in a matched-group design. As biopsy could lead to false negative results and imaging has been proven to reliably and safely identify local recurrence, we did not use the staged post-operative histology as was initially described by Desai and colleagues (25). However, our matched trial showed the promising oncologic results of LRC, and these results were comparable with those of the reference standard NSS for the treatment of small RCC during the intermediate term follow up period. Still, we do not think that our current data is sufficient to justify the use of cryoablation as a first-line option as partial nephrectomy remains the "gold standard" treatment. While cryoablation showed promising results as an alternative for treating small renal mass in patients with comorbidities or old age patients, more follow up is still required to expand the indications for performing cryoablation, and especially for the younger and healthy patients.
CONCLUSION
Compared with OPN, which is the reference standard for NSS, LRC with using ultra-thin cryoprobes for the treatment of small RCC showed similarly effective oncologic results during the intermediate term follow up in this matched trial. The LRC in our series revealed the merits of minimal invasiveness, less blood loss and a shorter hospital stay, together with a low incidence of treatment-related adverse events. Long term oncologic data on LRC is necessary to determine the definitive role of this procedure for treating RCC.