Introduction
With improved techniques for diagnostic surveillance, the incidence of early gastric cancers appears to be increasing, and stomach cancer is becoming a curable disease in many patients with early gastric cancer. In addition, the positive rate of lymph node metastasis in intramucosal gastric carcinomas (IGCs) is practically negligible. Thus, IGCs are the main candidate for minimally invasive surgical procedures such as endoscopic submucosal dissection (ESD). However, some intramucosal carcinomas do recur, and metastasize to regional lymph nodes and even to liver [1-4]. We recently experienced an unusual case of intramucosal gastric adenocarcinoma treated by complete ESD treatment, and the patient was found to have a widely disseminated metastatic disease after three years. In fluorescence in situ hybridization (FISH) study, both original and recurrent tumors harbored MET amplification.
Case Report
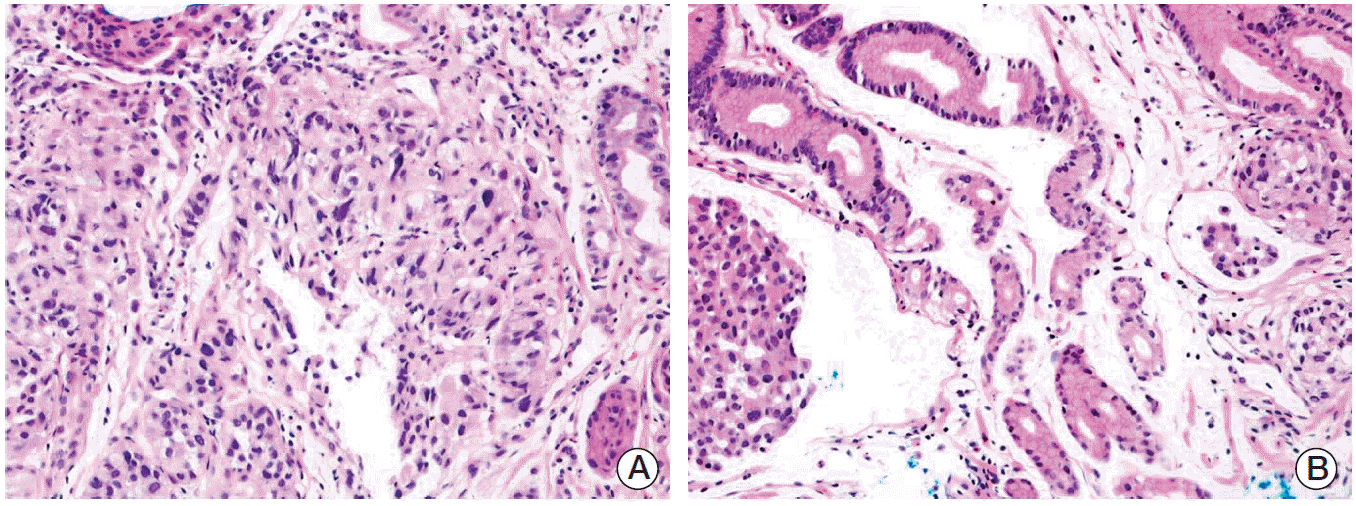
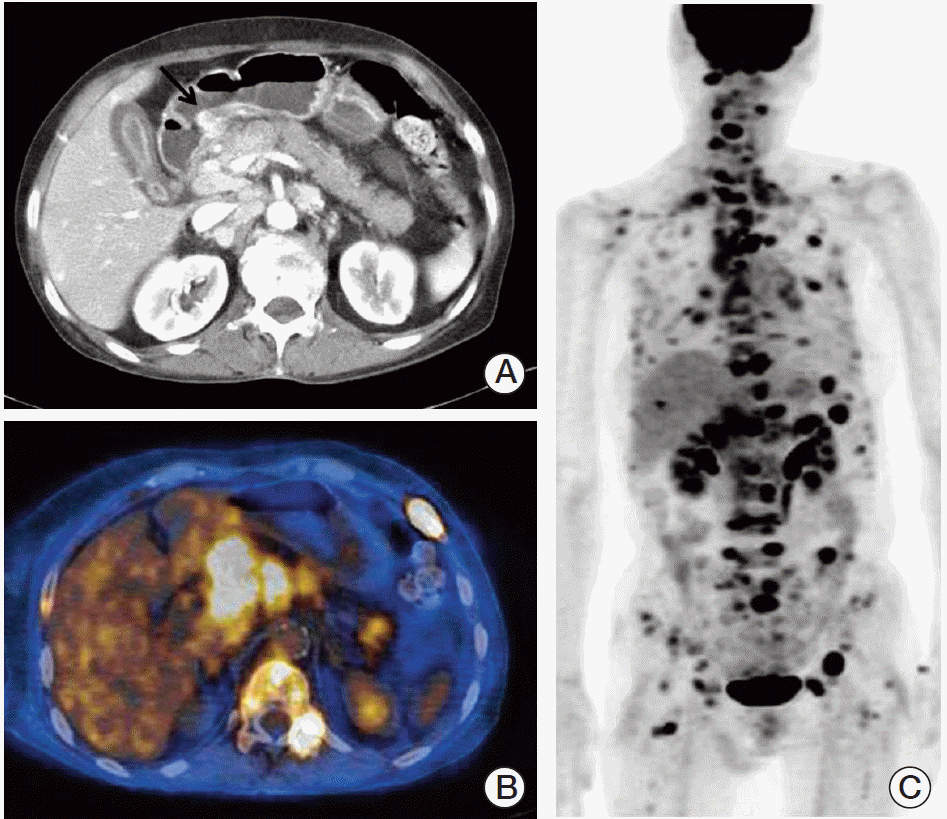
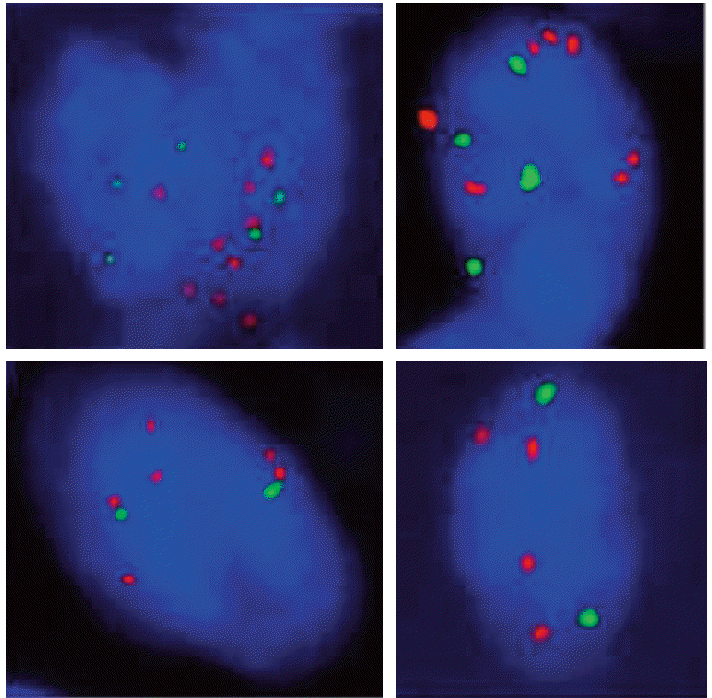
A 66-year-old female was admitted to the hospital for ESD with the diagnosis of early gastric cancer. She had been treated for rheumatoid arthritis for more than seven years. The lesion appeared to be shallow and computed tomography (CT) scan showed no evidence of regional and distant metastases, therefore, ESD was performed. Grossly, a slightly depressed lesion (type IIc) was observed in the center of the specimen from ESD. Histopathological examination revealed moderately differentiated adenocarcinoma, measuring 1.6 cm in diameter (Fig. 1). Focally, there were areas of poorly differentiated adenocarcinoma (less than 10% of the entire tumor volume). The tumor was confined to the mucosa, and the tumor did not invade into muscularis mucosa. No lymphatic or venous invasion was identified. The tumor was completely excised with clear, deep, and lateral resection margins. The patient was followed up with annual esophagogastroduodenoscopic examination and remained healthy without disease. Tumor recurrence was diagnosed on endoscopic biopsy three years after ESD (Fig. 2). This time, the tumor was mainly composed of poorly differentiated adenocarcinoma, and several lymphatic tumor emboli were observed even in the biopsy samples. Abdominal CT scan showed advanced gastric cancer on the antrum the same location of the initial adenocarcinoma (Fig. 3A). Positron emission tomography scan showed extensive lymph node and skeletal metastases (Fig. 3B and C). Because this case showed very aggressive biologic behavior in spite of no definite worse pathological parameters, we assumed that some specific genetic alterations might be present in this tumor. Then, we found five genes with prognostic and/or therapeutic implications in gastric cancers. These are MET [5,6], epidermal growth factor receptor (EGFR) [7], HER2 [8], fibroblast growth factor gene 2 (FGFR2) [9], and phosphatidylinositol 3-kinase catalytic subunit alpha (PIK3CA) [10]. For these genes, except HER2, we performed a FISH study in both original and recurrent tumors. We used MET/CEN 7, EGFR/CEN 7, FGFR2/CEN 10, and PIK3CA/CEN 3 dual color probes (ZytoVision, Bremerhaven, Germany). According to the National Comprehensive Cancer Network (NCCN) guidelines 2013, gene amplification was determined when the MET/chromosome 7 ratio was ≥ 2. If the ratio was < 1.8, it was considered negative, while ratio between 1.8 and 2 was considered borderline. The same criteria were applied to other genes. For HER2, we only performed immunohistochemical staining, since immunohistochemistry is well correlated with this gene amplification. As a result, we found that both original and recurrent tumors harbored MET amplification only (Fig. 4). The MET gene-to-chromosome ratio was 2.4 in the original tumor and 2.1 in the recurrent tumor. Low grade of polysomy 7 (two to four copies) was also noted in both tumors. Immunohistochemistry for HER2 was negative. Because the patient is inoperable, she only receives conservative treatment.
Discussion
Early gastric cancer, especially differentiated intramucosal carcinoma, poses a very low risk of lymph node metastasis, and is the main candidate for endoscopic treatment. However, practically, these tumors are not in the safety zone. They do metastasize, even though the number of such cases is very low. Korenaga et al. [11] reported 11 out of 568 cases (1.94%) of IGCs with lymph node metastatic disease, and Song et al. [4] reported that the incidence of metastatic IGC was 2% from a total of 1,981 cases. These authors suggested that pathological parameters such as diffuse type histology and muscularis mucosa invasion were deciding factors for lymph node metastasis. In the meantime, only a limited number of IGC cases have been reported for their extraordinarily aggressive clinical courses. Some IGCs metastasized to the ovaries, and clinically presented with Krukenberg tumors [12], while a single case of IGC was reported for nodal recurrence and liver metastasis even after curative ESD treatment [2]. Nonetheless, tumor recurrence with whole body dissemination, as presented in the present case, is unparalleled for its aggressiveness. Only three cases reported by Angeles-Angeles et al. [1] are comparable to our case, and all of these patients succumbed to the widely metastatic disease caused by IGC. The authors suggested that these unusual cases are a variant of IGC.
Originally, Yamao et al. [13] reported that the incidence of lymph node metastasis from IGC was 3.5%. They reported that lymphatic vessel invasion, histologic ulceration of the tumor, and larger size (≥ 30 mm) were independent risk factors predicting regional lymph node metastasis. They also found that the incidence of lymph node metastasis negative for these three factors was only 0.36% (one of 277 patients). Thus, patients whose tumor has these risk factors should be monitored more closely, or more invasive surgical techniques might be necessary for these patients. On the other hand, the present case is contrary to the general conceptions. The tumor size was relatively small (1.6 cm), there was no histological ulceration, most of the tumor consisted of differentiated adenocarcinoma, no lymphovascular invasion was observed, and the tumor did not even touch the muscularis mucosa. Poorly differentiated carcinoma component was found in the original tumor, but this element was so tiny (less than 2 mm) to be practically negligible. Nonetheless, this tumor showed recurrence, with wide dissemination throughout the body, even after curative endoscopic resection. This prompted our curiosity about the genetic background of this case, which revealed MET gene amplification.
MET is a proto-oncogene that encodes the receptor tyrosine kinase for hepatocyte growth factor (HGF). Regulated expression of MET plays a role in normal physiologic processes such as organ regeneration and wound healing, and there is a growing evidence that cancers with increased MET activity facilitate cell growth, protection from apoptosis, and invasion/metastasis [14]. In fact, overexpression of MET receptor and its HGF ligand has been reported in many of gastric cancers, and this has been uniformly associated with a more aggressive phenotype [15]. In line with this, Graziano et al. [5] recently reported the association of an increase in MET gene copy number with higher presenting tumor stage and grade as well as shorter median survival in stage II/III gastric adenocarcinomas. In addition, Lennerz et al. [6], who applied a strict standard for gene amplification by FISH, found that only 10 cases from 489 patients (2%) with advanced gastroesophageal cancers showed MET amplification. In this paper, patients with a MET-amplified tumor had significantly poorer survival than those with either EGFR or HER2 amplification, and the authors defined a MET-amplified tumor as a small but aggressive subgroup. However, since most of the previous cases that showed true MET amplification were advanced gastric cancers, this genetic amplification in early gastric cancers, particularly in IGCs has not received a lot of attention. We present such an exceptional case here, and this case provides an intriguing possible link of MET amplification with outrageous clinical behavior, even in the IGC. Although MET amplification alone may not explain the whole and there might be some unidentified complex mechanism, MET amplification was the only genetic alteration that we found after assessment of five genes, including EGFR, HER2, FGFR2, and PIK3CA. In addition, the current case did not have definite pathological parameters predicting this usually aggressive clinical course. In this circumstance, it appears reasonable that we assume that at least MET would play a critical role in the tumor biology of the present case. As previously indicated, we suggest that IGCs with unusually aggressive clinical behavior are a variant or a subset of IGCs, and MET amplification may define this subgroup among IGCs. In the current case, it is very unusual that there was no liver or lung metastasis, despite extensive lymph nodes and skeletal metastases. This remains to be explained.
In summary, we describe a very unusual case of IGC that showed recurrence and was disseminated throughout the body even after curative ESD treatment. Both original and recurrent tumors harbored MET amplification. As previously indicated, MET-amplified tumor may define an aggressive subgroup even in the IGCs. Expanded molecular study and clinical follow-up in a large group of IGCs will be necessary in order to investigate this matter.