AbstractPurposeThis study involved a population-based survey to provide evidence of public awareness of risk factors of gastric cancer and to investigate attitudes for the screening of gastric cancer in the South Korean population.
Materials and MethodsUsing a nationwide random selection method, 2014 subjects were enrolled in the study between 5 September 2006 and 25 September 2006.
ResultsIn terms of the awareness of risk factors, awareness was scored as the percentage of the probability of developing gastric cancer when a subject had a particular risk factor. For the risk factors, stress ranked highest with a score of 73.5%, followed by chronic gastritis (score of 72.1%), gastric ulcer (score of 71.2%) and a previous gastrectomy history (score of 68.7%). Other factors included a diet of charred foods (score of 67.3%), alcohol use (score of 65.3%), salty diet (score of 65.1%), history of smoking (score of 64.3%) and Helicobacter pylori infection (score of 57.5%). Subjects believed that 60.4% of all gastric cancers were preventable by lifestyle modification and the subjects believed that regular screening could prevent 72.1% of all gastric cancers. However, 54% of subjects did not receive regular screening and the most common reason for not undergoing screening was a lack of symptoms.
IntroductionAlthough the incidence of gastric cancer has been decreasing gradually worldwide, gastric cancer is still the second most common leading cause of cancer death in the world and populations in Korea and Japan have the greatest risk of developing gastric cancer as compared to any other known population (1). Geographic differences in the incidence of gastric cancer have been ascribed to several reasons, and environmental factors have a greater influence on gastric cancer development than genetic factors (2,3). Of the environmental factors, dietary factors are probably responsible for the regional differences (4). In addition, Helicobacter pylori infection has been associated with gastric cancer, and infection rates and infecting bacterial strain types differ in Asia and other world regions (5).
Two approaches have been used to reduce cancer mortality. One approach is primary prevention to reduce the development of cancer by eliminating risk factors and a second approach for prevention is early detection. It is well known that an early stage gastric cancer has a good prognosis.
For primary prevention of gastric cancer, public awareness of the risk factors of gastric cancer and efforts to modify risk factors are needed. Numerous lessons have been learned from studies of other cancers (6,7). For cancers such as breast cancer, colorectal cancer and cervical cancer, a lack of awareness of risk factors and a lack of perception of self-risk may be reasons for apparent lack of concern (8,9). However, no systematic studies have been undertaken of these issues for gastric cancer. In particular, informative studies are not available on the level of public awareness of risk factors or perception of self-risk.
For secondary prevention of gastric cancer, regular screening is a prerequisite. In South Korea, it has been recommended that all persons older than 40 years should receive regular screening every two years regardless of symptoms, but the actual examination rate is not satisfactory. Thus, reasons for low screening rates should be determined to aid the planning of future strategies aimed at gastric cancer prevention. For other cancers, such as breast, colon, lung and cervical cancer, many systematic studies about views of general subjects on preventative measures have been undertaken, especially in Western countries (10,11). However, comparatively little is known about how the general population decides to adopt preventative measures of gastric cancer.
We undertook this study to provide evidence for public awareness of gastric cancer risk factors and for the perception of self-risk of gastric cancer development in a representative population in South Korea. In addition, we have investigated attitudes of the South Korean general population regarding gastric cancer screening. As we have been interested in the quantitative estimation of public awareness, we have emphasized awareness of attributable and preventable risks, defined as the percentage contribution made by a factor to the risk of developing gastric cancer, or as the percentage reduction in disease incidence expected if a factor was eliminated.
Materials and MethodsThe present study involved a population-based survey. Using a nationwide random selection method based on recruiting numbers by region, 2014 subjects aged 19 years or older were enrolled between 5 September 2006 and 25 September 2006. Trained interviewers with the use of a face-to-face written interview conducted the survey. Informed consent was obtained from all participants prior to interviews.
The questionnaire used in the survey contained questions on the following issues (Table 1):
Statistical analysis was conducted to identify different parameters using Pearson's chi-squared test with the use of a statistical software package (SPSS for Windows, version 12.0; SPSS, Chicago, IL). Significance was set at p<0.05. Multivariate regression analysis was also performed to investigate correlations between significant demographic parameters and special risk factors. We used the Scheffe method when the post-hoc test was conducted.
Results2. Estimation of perceived self-risk of gastric cancerPerceptions of gastric cancer self-risk are summarized in Fig. 1. Among all subjects, 40.1% of the subjects believed that their self-risk of gastric cancer was average, 36.3% of the subjects believed that their self-risk was low and 9.7% of the subjects believed that their self-risk was very low. However, 9.8% of the subjects believed that they had a high risk to develop gastric cancer and 1.2% of the subjects considered that the risk was very high.
The characteristics of the subjects found to influence perception of gastric cancer self-risk were the following: age (p=0.03), education (p=0.04), region of residence (p<0.01), marriage (p<0.01), cancer insurance (p=0.02), current health status (p<0.01), smoking (p=0.03), alcohol use (p<0.01), salty diet (p<0.01), and a spicy diet (p=0.04). Data including post-hoc test findings is summarized in Table 3.
Sixty percent of subjects with a good self-assessed current health status considered that their risk of gastric cancer was low or very low, but only 23.2% of subjects with a poor self-assessed health status considered that their risk of gastric cancer was low or very low (p<0.01). Seventeen percent of current smokers thought that their self-risk of gastric cancer was high or very high, but only 11.2% of non-smokers thought that their self-risk of gastric cancer was high or very high (p=0.03).
According to alcohol intake, 51% of non-users thought that their risk of developing gastric cancer was low or very low, but 42.2% of those that consumed alcohol every week thought that their risk of developing gastric cancer was low or very low. Interestingly, 49.4% of subjects that consumed alcohol daily thought that their risk of developing cancer was low or very low (p<0.01).
3. Public awareness of risk factors associated with gastric cancerTo determine public awareness of risk factors associated with gastric cancer, we asked subjects whether certain items were risk factors of gastric cancer. Subjects were asked to score answers between 0 and 100, where "0" meant no association and "100" represented certainty. The results are summarized in Fig. 2.
Stress was regarded as the most powerful risk factor. Subjects believed that the probability of development of gastric cancer due to stress was 73.5% (95% confidence interval-CI: 72.7~74.4%), followed by the presence of gastric lesions, including chronic gastritis, a gastric ulcer and a previous gastrectomy history (72.1%; 95% CI: 71.3~73.0%), 71.2% (95% CI: 70.3~72.0%) and 68.7% (95% CI: 66.9~68.8%), respectively.
Subjects believed that the probability of development of gastric cancer due to a diet of charred foods was 67.3% (95% CI: 66.4~68.2%) and a salty diet was 65.1% (95% CI: 64.2~66.0%).
The probability of development of gastric cancer caused by alcohol consumption and smoking were 65.3% (95% CI: 64.4~66.2%) and 64.3% (95% CI: 63.3~65.3%), respectively, and the probability of development of gastric cancer due to obesity and physical inactivity was 61.4% (95% CI: 60.4~62.3%) and 58.0% (95% CI: 57.1~58.9%), respectively. In terms of dietary factors, consumption of processed ham or sausages and a fatty diet ranked lower as compared to the above-mentioned factors. The probability of gastric cancer development due to Helicobacter pylori was 57.5% (95% CI: 56.5~58.5%).
As risk factors, age (48.7%, 95% CI: 47.7~49.7%) and sex were ranked lowest, except for the consumption of vegetables and fruits. The risk of gastric cancer based on a family history of gastric cancer was 61.0% (95% CI: 60.0~62.0%).
4. Awareness of preventable risks of gastric cancerTo determine individual awareness of preventable factors, we asked subjects what percentage of gastric cancers would be prevented if a particular given risk factor was totally removed. Results are summarized in Fig. 2.
The study population considered that 68.9% of gastric cancers could be prevented by eliminating stress (95% CI: 67.9~69.8%). The participants believed that the elimination of a diet of charred foods would reduce the incidence of gastric cancer by 59.2% (95% CI: 58.3~60.1%) and the elimination of a salty diet would reduce the incidence of gastric cancer by 57.6% (95% CI: 56.7~58.5%).
As compared with the dietary factors, treatment of gastric lesions including gastric ulcers, chronic gastritis and a previous gastrectomy history was regarded as a more effective measure to reduce the risk of gastric cancer as compared to the risk due to dietary factors. The corresponding reductions believed possible by eliminating these three types of gastric lesions were 66% (95% CI: 65.1~66.9%), 66.7% (95% CI: 65.8~67.6%) and 65.1% (95% CI: 64.1~65.9%), respectively.
The elimination of alcohol consumption and smoking were considered to prevent 60.4% (95% CI: 59.4~61.3%) and 59.3% (95% CI: 58.2~60.3%) of gastric cancers.
5. Awareness of genetic factors and the value of lifestyle modificationsSubjects considered that the proportion of genetic contribution to the development of gastric cancer was 38.5%. Women (40.1% versus 36.8% for men, p<0.01) and homemakers (41.6% versus 37.7% for working women, p=0.04) were more likely to believe that gastric cancer was genetically determined. Current health status also influenced opinions Subjects with a good self-assessed current health status considered that the risk of gastric cancer determined genetically was 37.4%, whereas the risk was 41.3% in subjects with a poor current health status (p<0.01).
Subjects considered that 60.4% of gastric cancers were preventable by lifestyle modification. Results concerning smoking were interesting; current smokers believed that 59.4% of gastric cancers could be prevented by lifestyle modification and non-smokers believed that 60.4% of gastric cancers could be prevented, whereas ex-smokers thought that 63.6% of gastric cancers were preventable by lifestyle modification (p=0.03). Other factors such as education, occupation, and region of residence were not significant.
6. Perception for the values of early diagnosis and regular screeningThe distribution of subjects according to the perception of gastric cancer curability for an early diagnosis is shown in Fig. 3. Among all subjects, 29.5% considered that early gastric cancer curability was 80~89%, 24.5% of the subjects thought early gastric cancer curability to be 90~99% and 7.9% of the subjects believed that early gastric cancer was completely curable.
In terms of the value of regular screening, 61.4% of the subjects considered that regular screening was very helpful for early detection and 37.6% of the subjects thought that regular screening was helpful. Only 1% of subjects considered that regular screening was unhelpful. Moreover, the subjects believed on average that 72.1% of gastric cancers were preventable by regular screening. Subject characteristics, such as education, occupation, income and current health status were not found to influence significantly perceptions concerning regular screening.
In terms of screening, 46% of subjects had undergone screening for gastric cancer at least once. Age (p<0.01), a lower education status (p<0.01), a lower family income (p<0.01), married status (p<0.01), poor current health (p<0.01), ex-smoking status (p<0.01), heavy alcohol consumption (p<0.01) and not favoring a spicy diet (p=0.01) or a fatty diet (not favoring, p<0.01) were found to influence adversely subject behavior in terms of screening. Of the subjects that had undergone screening, 54.2% of the subjects had undergone screening within one year, 21.8% of the subjects had undergone screening within the past 1~2 years and 11.9% of the subjects had undergone screening within the past 2~3 years, 6.8% of the subjects had undergone screening within the past 3~5 years and 5.3% of the subjects had undergone screening prior to five years.
Of those subjects who did not receive regular screening, reasons for not undergoing screening are shown in Table 4. The most common cause of not receiving regular screening was the absence of symptoms. Future plans in terms of screening for gastric cancer are shown in Table 5. Among all respondents, 22.2% had a screening plan when they developed symptoms and 10.9% of respondents had no screening plan.
DiscussionTo reduce gastric cancer mortality, reversible risk factors should be modified and regular screening should be performed (12). To achieve this goal, public education should be undertaken as a high level of public awareness of risk factors is required. There are studies on the differences between the cancer risk perceptions by the general population and their behavior towards the prevention of cancer (13-17). Therefore, it is important to determine current levels of risk factor awareness and perceptions of screening programs intended for the general population.
However, the extent of public awareness of the risk factors of gastric cancer has not been well studied. This is also the case in South Korea that has the highest incidence of gastric cancer in the world. The present study is the first large-scale study conducted in South Korea and was undertaken to assess public awareness of risk factors of gastric cancer. As far as we are aware, no previous study has been undertaken to gauge the level of public awareness of gastric cancer.
Several risk factors of gastric cancer have been described. Although genetic and environmental factors are involved in the development of gastric cancer, it appears that environmental factors are more important (2,3). Thus, changing or eliminating environmental risk factors could reduce gastric cancer incidence, but require essential lifestyle modifications in the general population (18,19).
In our study, the Korean subjects thought that genetic predisposition accounted for 38.5% of gastric cancers and 61.5% of gastric cancers were regarded to be caused by environmental factors. In addition, the Korean subjects believed that 60.4% of gastric cancers were preventable by lifestyle modifications.
As shown in the present study, estimations of gastric cancer self-risk were not high. Only 11.0% of the study subjects thought that they had a high or very high risk of developing gastric cancer. Forty-six percent of the study subjects believed they had a low or very low risk of gastric cancer. This finding indicates that the subjects were not over-concerned with the self-risk of gastric cancer and is reflected by participation in screening programs. Fifty-four percent of the Korean subjects have never undergone a check-up for gastric cancer, and most frequently cited a lack of symptoms as the cause of not receiving screening. To facilitate regular screening, the public should be educated concerning the need for regular screening regardless of symptoms.
In the present study, we investigated both public awareness of 'attributable risk' and 'preventable risk'. The 'attributable risk' associated with factor 'A' is the risk of gastric cancer development associated with the presence of this risk factor. However, during the development of gastric cancer, several risk factors are likely to be involved, and the 'preventable risk' associated with factor 'A' is the probability of preventing gastric cancer development by eliminating factor 'A'. Thus, there may be a gap between the 'attributable' and 'preventable' risk associated with the same risk factor.
For questions concerning 'attributable risks', stress was regarded as being the most powerful risk factor, and the study subjects believed that the probability to develop gastric cancer when they were under stress was 73.5%. In addition, dietary factors were viewed as being less important than stress or gastric lesions, such as a gastric ulcer, chronic gastritis or previous gastrectomy. Recently, infection with Helicobacter pylori has received much publicity and the general population has been aware of the effects of bacterial infection. However, the probability of development of gastric cancer due to Helicobacter pylori was scored as 57.5%, which was the lowest score (except for effects of air pollution, age and sex).
Regarding questions about the awareness of 'preventable risks', stress was also ranked highest followed by the presence of gastric lesions (gastric ulcer, chronic gastritis and a previous gastrectomy history), alcohol use, Helicobacter pylori infection and smoking. The study population considered that elimination of stress reduces the risk of gastric cancer development by 68.9%. The elimination of a diet of charred foods and a salty diet were believed to reduce the risk of gastric cancer development by 59.2% and 57.6%, respectively. As compared with these dietary factors, treatment of gastric lesions such as a gastric ulcer, chronic gastritis and a history of gastrectomy was regarded as being more capable to reduce the risk of gastric cancer development.
Interestingly, stress was ranked highest in terms of attributable and preventable risks. The subjects believed that stress was most strongly related to gastric cancer development and that gastric cancer could be prevented most effectively by stress reduction.
In terms of the causal relationship between stress and cancer, much research has been carried out for breast cancer, but studies on the relationship between stressful life events and breast cancer risk have produced conflicting results. In one cohort study, the hazard ratio for breast cancer per one stressful event increase was determined as 1.07 (95% CI: 1.00~1.15), whereas the hazard ratio of divorce and separation was 2.26 (95% CI: 1.25~4.07) and that of the death of a husband was 2.00 (97% CI: 1.03~3.88) (20). However, in another prospective cohort study, which was conducted in Finland, no evidence of any association between self-perceived daily stress and breast cancer risk was found (21), and a meta-analysis conducted by Duijts et al. failed to support an overall association between stressful events and breast cancer risk (22). In addition, Nielsen et al. concluded that stress does not appear to increase the risk of breast cancer (23). For colorectal cancer, several studies have examined the relationship between stress and cancer risk. In one study, a history of serious work-related problems during the prior 10 years was found to be strongly associated with the occurrence of colorectal cancer (odds ratio, 5.5; 95% CI: 2.3~23.5) (24).
However, for gastric cancer, few studies have examined the relationship between stress and gastric cancer risk. According to a nationwide Swedish case-control study conducted by Catarina and colleagues, work-related stress does not appear to be important in the etiology of gastric cancer. However, significant associations have been reported between job strain and the risk of gastric cardia cancer (odds ratio, 2.2; 95% CI: 1.0~4.8) and between a covert coping style and gastric cancer (odds ratio, 1.8; 95% CI: 1.0~2.3) (25). Thus, the relationship between stress and gastric cancer risk requires further clarification.
The findings of the present study encourage us to make several suggestions. First, accurate information about the risk factors of gastric cancer should be presented to the general population. For example, Helicobacter pylori infection, smoking, and dietary factors need to be emphasized at the expense of obesity, air pollution and stress. Second, lifestyle modifications are to be encouraged based on an awareness of risk factors, and third, more accurate information about screening should be presented to the general population, especially on the need for screening regardless of symptoms.
This study has some limitations. First, the questions were somewhat subjective. For example, a 'salty diet' or 'spicy diet' is not well defined, so the respondents may have different concepts on the definition. A more objective method would be to define the use of 'salty' or 'spicy' in quantitative terms. Second, favoring a diet is also somewhat subjective. More objective methods are needed to define the diet pattern; for example, how often the respondents ate salty or spicy foods. Third, the concept of 'stress' is also broad. Stress can refer to many events with various levels of stress. However, the aim of this study was to assess the level of awareness of gastric cancer risk factors in the general population and to determine the association of awareness and the behavior of the general population. Thus, simple questions were employed.
The present study provides evidence of the public awareness of risk factors of gastric cancer and perceptions of self-risk in a population at particularly high risk. In particular, the quantitative determination of the awareness of attributable and preventable risks of risk factors in the present study is unique. In addition, this study reveals the attitudes of South Korean subjects to gastric cancer screening.
References1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. PMID: 15761078
2. Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. PMID: 16489633
3. Forman D, Burley VJ. Gastric cancer: global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633–649. PMID: 16997150
4. Shikata K, Kiyohara Y, Kubo M, Yonemoto K, Ninomiya T, Shirota T, et al. A prospective study of dietary salt intake and gastric cancer incidence in a defined Japanese population: the Hisayama study. Int J Cancer. 2006;119:196–201. PMID: 16450397
5. Gwack J, Shin A, Kim CS, Ko KP, Kim Y, Jun JK, et al. CagA-producing Helicobacter pylori and increased risk of gastric cancer: a nested case-control study in Korea. Br J Cancer. 2006;95:639–641. PMID: 16909137
6. Keighley MR, O'Morain C, Giacosa A, Ashorn M, Burroughs A, Crespi M, et al. Public awareness of risk factors and screening for colorectal cancer in Europe. Eur J Cancer Prev. 2004;13:257–262. PMID: 15554552
7. Pohls UG, Renner SP, Fasching PA, Lux MP, Kreis H, Ackermann S, et al. Awareness of breast cancer incidence and risk factors among healthy women. Eur J Cancer Prev. 2004;13:249–256. PMID: 15554551
8. Peacey V, Steptoe A, Davidsdottir S, Baban A, Wardle J. Low levels of breast cancer risk awareness in young women: an international survey. Eur J Cancer. 2006;42:2585–2589. PMID: 16829071
9. Powe BD, Finnie R, Ko J. Enhancing knowledge of colorectal cancer among African Americans: why are we waiting until age 50? Gastroenterol Nurs. 2006;29:42–49. PMID: 16552299
10. Ackermann S, Renner SP, Fasching PA, Poehls U, Bender HG, Beckmann MW. Awareness of general and personal risk factors for uterine cancer among healthy women. Eur J Cancer Prev. 2005;14:519–524. PMID: 16284496
11. McMenamin M, Barry H, Lennon AM, Purcell H, Baum M, Keegan D, et al. A survey of breast cancer awareness and knowledge in a Western population: lots of light but little illumination. Eur J Cancer. 2005;41:393–397. PMID: 15691638
12. Adami HO, Day NE, Trichopoulos D, Willett WC. Primary and secondary prevention in the reduction of cancer morbidity and mortality. Eur J Cancer. 2001;37(Suppl 8):S118–S127. PMID: 11602378
13. Salant T, Ganschow PS, Olopade OI, Lauderdale DS. "Why take it if you don't have anything?" Breast cancer risk perceptions and prevention choices at a public hospital. J Gen Intern Med. 2006;21:779–785. PMID: 16808782
14. Sabates R, Feinstein L. The role of education in the uptake of preventative health care: the case of cervical screening in Britain. Soc Sci Med. 2006;62:2998–3010. PMID: 16403597
15. Wee CC, McCarthy EP, Phillips RS. Factors associated with colon cancer screening: the role of patient factors and physician counseling. Prev Med. 2005;41:23–29. PMID: 15916989
16. Macrae FA, Hill DJ, St John DJ, Ambikapathy A, Garner JF. Predicting colon cancer screening behavior from health beliefs. Prev Med. 1984;13:115–126. PMID: 6718327
17. Keighley MR, O'Morain C, Giacosa A, Ashorn M, Burroughs A, Crespi M, et al. Public awareness of risk factors and screening for colorectal cancer in Europe. Eur J Cancer Prev. 2004;13:257–262. PMID: 15554552
18. Larsson SC, Bergkvist L, Wolk A. Fruit and vegetable consumption and incidence of gastric cancer: a prospective study. Cancer Epidemiol Biomarkers Prev. 2006;15:1998–2001. PMID: 17035412
19. Gajalakshmi CK, Shanta V. Lifestyle and risk of stomach cancer: a hospital-based case-control study. Int J Epidemiol. 1996;25:1146–1153. PMID: 9027518
20. Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, Koskenvuo M. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am J Epidemiol. 2003;157:415–423. PMID: 12615606
21. Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, Koskenvuo M. Stress of daily activities and risk of breast cancer: a prospective cohort study in Finland. Int J Cancer. 2001;91:888–893. PMID: 11275996
22. Duijts SF, Zeegers MP, Borne BV. The association between stressful life events and breast cancer risk: a meta-analysis. Int J Cancer. 2003;107:1023–1029. PMID: 14601065
23. Nielsen NR, Gronbaek M. Stress and breast cancer: a systematic update on the current knowledge. Nat Clin Pract Oncol. 2006;3:612–620. PMID: 17080179
24. Courtney JG, Longnecker MP, Theorell T, Gerhardsson de Verdier M. Stressful life events and the risk of colorectal cancer. Epidemiology. 1993;4:407–414. PMID: 8399688
25. Jansson C, Johansson AL, Jeding K, Dickman PW, Nyren O, Lagergren J. Psychosocial working conditions and the risk of esophageal and gastric cardia cancers. Eur J Epidemiol. 2004;19:631–641. PMID: 15461194
Fig. 2Awareness of attributable and preventable risks in gastric cancer. An 'attributable risk' associated with factor 'A' is the risk of gastric cancer development associated with the presence of this risk factor. However, during the development of gastric cancer, several risk factors are likely to be involved. 'Preventable risk' associated with factor 'A' is the probability of preventing gastric cancer development by eliminating factor 'A' (100%: gastric cancer can be prevented totally if risk factor 'A' is removed; 50%: 50% of gastric cancer can be prevented if risk factor 'A' is removed). 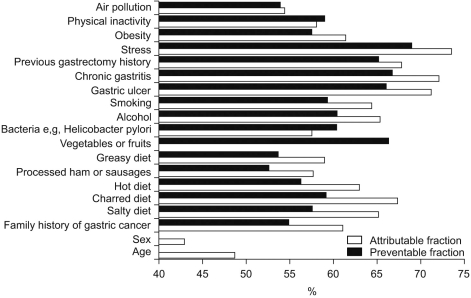
Table 3Perception of self-risk of gastric cancer  *assigned score 5: very low, 4: low, 3: average, 2: high, 1: very high. Calculated mean values of sums. †p<0.05 as compared with age 19~29 years by the Scheffe Post-hoc test, ‡p<0.05 as compared with middle school by the Scheffe Post-hoc test, §p<0.05 as compared with town by the Scheffe Post-hoc test, ∥p<0.05 as compared with good by the Scheffe Post-hoc test, ¶p<0.05 as compared with never-smoker by the Scheffe Post-hoc test, **p<0.05 as compared with never by the Scheffe Post-hoc test, ††p<0.05 as compared with favor by the Scheffe Post-hoc test, ‡‡p<0.05 as compared with favor by the Scheffe Post-hoc test. |
|
||||||||||||||||||||||||||||||||||||||||