AbstractPurposeThe purpose of the present study was to assess the biological effects of TNF-alpha in Caco-2 well-differentiated colon adenocarcinoma cells and to determine radiation sensitivity in order to develop TNF-alpha into a cancer therapeutic agent.
Materials and MethodsA cell viability test was conducted via a colorimetric and colony forming assay after 1 day and 3 days of incubation with TNF-alpha. Western blotting analysis and immunofluorescence staining were conducted to explore TNF-alpha-induced morphological and molecular changes in the adhesion molecules, E-cadherin and claudin-4. The effects of γ-irradiation at a dose of 2 Gy on cell survival were evaluated by a clonogenic assay. The molecular changes in apoptosis-regulatory proteins were assessed by Western blotting.
ResultsCaco-2 cells were highly resistant to TNF alpha-induced cell death and 2 Gy of γ-irradiation. However, we observed the downregulation of the adherens junctional protein, E-cadherin and translocation of tight junctional protein, claudin-4 from the membrane to the cytosol induced by TNF-alpha treatment which would indicate cell-cell junction disruptions. These alterations of junctional proteins influenced the regulation of cell death in response to 2 Gy of γ-irradiation. The combined treatment of TNF-alpha with 2 Gy of γ-irradiation reduced the survival of Caco-2 cells by down-regulating bcl-xl and activating JNK pathways.
IntroductionTNF-alpha, an inflammatory cytokine, can induce a diverse range of biological responses (1-3). Signaling by TNF-alpha is initiated through binding to its receptor, tumor necrosis factor receptor 1 which induces apoptosis via caspase-8 and caspase-3 activation in many cancer cells (4,5). TNF-alpha has been previously proposed as a possible candidate for cancer treatment because TNF-alpha enhanced LNCaP prostate cancer cells in response to radiation by ceramide-mediated apoptosis (6) and sensitized the Hodgkin cell line, HD-MyZ, to chemotherapeutic drugs through the activation of caspase-3 and Bid cleavage (7). However, TNF-alpha signaling also induces anti-apoptotic signals by inducing tumor growth mediated by NF-kB transcription factor (8).
Alteration of junctional proteins has been reported in case studies with TNF-alpha treatment applied to cancer cells (9-11) as well as endothelial cells (12,13). In the case of endothelial cells, enhanced vascular permeability by TNF-alpha treatment facilitates the uptake of chemotherapeutic drugs and antibodies and contributes to enhanced apoptosis (14,15). However, TNF-alpha-induced alterations of junctional proteins do not contribute to apoptosis in the majority of cancer cells, and this is particularly true in colon cancer cells (4). Only the combined treatment of TNF-alpha and interferon-γ disrupted barriers in T84 colonic epithelial cells and enhanced apoptosis (9).
Therefore, in this study, our intent was to determine the effects of TNF-alpha on the viability of Caco-2 well-differentiated colon adenocarcinoma cells, and to estimate the sensitivity of these cells to radiation in order to develop TNF-alpha into a therapeutic agent for cancer.
Materials and Methods1. Cell culture and TNF-alpha treatmentThe Caco-2 human colon adenocarcinoma cell line was obtained from the American Type Culture Collection (Manassas, VA) and maintained in DMEM containing 10% fetal bovine serum (FBS) with 100,000 U/L of penicillin and 100 mg/L of streptomycin (Gibco BRL, Gaithersburg, MD). The concentration of TNF-alpha (R&D Systems, Minneapolis, MN) used for treatment was 10 and 100 ng/mL respectively. For co-treatment of Caco-2 cells with TNF-alpha and γ-irradiation, 10 ng/mL of TNF-alpha was added 16 hr prior to exposing the cells to 2 Gy of γ-irradiation.
2. Cytotoxicity assayCell viability was evaluated by a colorimetric assay using a cell viability and cytotoxicity assay kit (Dojindo Molecular Technologies, Rockville, MD). Briefly, Caco-2 cells were seeded at 5×103 cells in 96-well plates and permitted to attach to the plates for 2 days after which TNF-alpha was added. After 3 days of incubation, 10 µL of color reagent was added to each well, and the cells were incubated for 1 hour. The mean absorbance at 450 nm in each set of samples was determined.
3. Clonogenic assayTo determine Caco-2 cell survival after exposure to γ-irradiation, 200 cells were seeded in 35 mm dishes and exposed to 2 Gy, 4 Gy and 8 Gy of irradiation in the presence or absence of TNF-alpha. After 10 days of incubation, the colonies were stained with crystal violet and counted. TNF-alpha was added 16 hr before irradiation to cells.
4. Western blot analysisTotal cell lysates were prepared for Western blot analysis. Caco-2 cells were harvested and lysed in a buffer containing 1% SDS, 1% Triton X-100, 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 µg/mL leupeptin, and 2 mM PMSF. 20 µg of total extracted proteins were applied per lane for SDS-PAGE. After transfer to nitrocellulose membranes, protein expression levels were assessed using specific antibodies to E-cadherin, PARP, bcl-xl, phospho-JNK (Cell signaling Technology, Danvers, MA), claudin-4 (Zymed, South San Francisco, CA), and α-tubulin (Sigma, St. Louis, MO).
5. Immunofluorescence staining and confocal microscopyImmunofluorescence staining was conducted on Caco-2 cells cultured on cover slips. All staining procedures were performed on ice or at 4℃ unless otherwise noted. The cells were fixed in 4% paraformaldehyde, permeabilized for 5 min with 0.1% Triton X-100, and antibodies were added at dilutions recommended by the manufacturer. Laser scanning confocal microscopy was done using a MRC-1024/ES confocal microscope (Bio-Rad, Hercules, CA).
Results1. Caco-2 cells are resistant to TNF-alpha-induced cell deathIn an effort to assess the biological effects of TNF-alpha, we first determined the viability and cytotoxicity of TNF-alpha in Caco-2 cells. From the cytotoxicity assay, Caco-2 cells were shown to be highly resistant to TNF-alpha-induced cell death and viable in the presence of TNF-alpha for 3 days at 10 ng/mL and 100 ng/mL concentrations, respectively (Fig. 1A). No significant changes in cell survival were observed by increasing the concentration of TNF-alpha up to 10-fold. We used 10 ng/mL of TNF-alpha for all further experiments looking at the effects of TNF alpha on Caco-2 cells.
2. TNF-alpha alters E-cadherin and claudin-4 in Caco-2 cellsAlthough Caco-2 cells are resistant to TNF-alpha-induced cell death even at high concentrations of TNF-alpha, molecular alterations of E-cadherin and claudin-4 were seen in Caco-2 cells. Down-regulated E-cadherin expression was detected via Western blot and Immunofluorescence staining. For claudin-4 the most abundantly expressed protein in the claudin family group of proteins in Caco-2 cells, the expression level remained the same, but the cellular localization of claudin-4 was altered from the membrane to the cytoplasm in a scattered pattern (Fig. 1B, C). This suggests disruptions of adherens junctional complexes and tight junctions in TNF-alpha-treated Caco-2 cells. The molecular alterations of E-cadherin and claudin-4 were neither accompanied by morphological changes or viability, but may influence susceptibility to cell death by secondary apoptotic stimuli. We used γ-irradiation as an apoptotic stress and applied various doses of γ-irradiation to the Caco-2 cells after treatment with TNF-alpha.
3. TNF-alpha enhances cell death induced by 2 Gy of γ-irradiationThe induction of cell death by 2 Gy of γ-irradiation, a relatively low dose of γ-irradiation, in TNF-alpha-treated Caco-2 cells was estimated by clonogenic assay for 10 days. There were no significant changes in the surviving fraction of TNF-alpha-treated Caco-2 cells under normal conditions or when exposed to 0 Gy or the control exposed to 2 Gy of γ-irradiation. However, we observed a significant reduction in the surviving fraction of Caco-2 Cells when pre-treated with TNF-alpha and exposed to 2 Gy of γ-irradiation (Fig. 2A). Only a 10% of reduction was observed in TNF-alpha-treated Caco-2 cells under normal condition and a similar result was obtained from the cytotoxicity assay (described in Fig. 1A). In addition, the control exposed to 2 Gy of γ-irradiation resulted in an 11% of reduction in the surviving fraction. Also, Caco-2 cells proved resistant to a low dose of γ-irradiation (2 Gy). This shows that neither TNF-alpha nor 2 Gy of γ-irradiation independently exert significant effects on cell death and survival in Caco-2 cells. However, the surviving fraction in cells exposed to 2 Gy of γ-irradiation following pre-treatment with TNF-alpha for 16 hr was reduced up to 50%. This suggests that pre-treatment with TNF-alpha combined with exposure to 2 Gy of γ-irradiation successfully enhanced cell death in Caco-2 cells.
In order to prove that 2 Gy of γ-irradiation with TNF-alpha pretreatment is sufficient to enhance cell death in Caco-2 cells, we applied various doses of γ-irradiation to Caco-2 cells after a 16 hr pre-treatment of TNF-alpha. After 10 days of clonogenic assay, significant alteration in the surviving fraction was detected only in cells under the 2 Gy of γ-irradiation condition (Fig. 2B). The surviving fractions in the control exposed to 4 Gy and 8 Gy of γ-irradiation were already reduced to 50% and 25%, respectively. The pre-treatment of TNF-alpha along with exposure to 4 Gy and 8 Gy of γ-irradiation did not enhance cell death of Caco-2 cells. Both 4 Gy and 8 Gy of γ-irradiation were too strong to discriminate influences of TNF-alpha on cell death from γ-irradiation stimuli. Therefore, we speculate that TNF-alpha could be applied as a radiosensitizer to enhance of γ-irradiation-induced cell death induced by a low dose such as 2 Gy and TNF-alpha-mediated alterations of adhesion molecules may facilitate γ-irradiation-mediated cell death in Caco-2 cells. Since a 50% reduction of the surviving fraction in response to 2 Gy of γ-irradiation in combination with the pretreatment of TNF-alpha was observed, we wanted to investigate molecular alterations in apoptosis-regulating proteins.
4. TNF-alpha activates pro-apoptotic signals in Caco-2 cellsIn order to elucidate the key regulatory molecules in cell death induced by combined treatment with TNF-alpha and 2 Gy of irradiation, the important regulators in cell death were determined by Western blot. Because bcl-2 family members are important to the induction of anti-apoptotic signals and the ectopic expression of anti-sense bcl-xl enhanced apoptosis in Caco-2 cells (17), the cleavage of PARP and levels of bcl-xl expression were assessed. Neither TNF-alpha nor 2 Gy of irradiation alone initiated proapoptotic signals via the downregulation of bcl-xl expression levels. There was a slightly increased in PARP cleavage although this effect was not sufficient for the induction of apoptotic cell death (Fig.3A). However, synergistic downregulation of bcl-xl and upregulation of cleaved PARP were noted in the combined treatments of TNF-alpha and 2 Gy of γ-irradiation. This correlates well with the enhanced cell death observed in the clonogenic assay (described in Fig. 2A).
5. TNF-alpha induces activation of JNK under 2 Gy of γ-irradiationBecause c-Jun N-terminal kinases (JNKs) are involved in cell death induced by environmental stress, we wanted to know how stress-activated JNKs are involved in the enhanced cell death in caco-2 cells induced by pre-treatment with TNF-alpha and exposure to 2 Gy of γ-irradiation. The activation of JNK-1, 2 and 3 were observed by Western blot with phospho-specific JNK antibodies in pre-treated TNF-alpha Caco-2 cells 6 hr after irradiation. Weak phosphorylation of JNK-1 was seen in both TNF-alpha-treated Caco-2 cells and cells exposed to 2 Gy of γ-irradiation. As we had anticipated, enhanced phosphorylation of JNK-1, 2 and 3 were observed in the combined treatment with TNF-alpha and 2 Gy of γ-irradiation (Fig. 3B). This correlates well with increases in cell death when using the combined treatment of TNF-alpha and 2 Gy of γ-irradiation in Caco-2 cells.
DiscussionTNF-alpha has been shown to induce an increase in tight-junctional permeability in Caco-2 cells; however, the molecular mechanisms responsible for these events and effects on cell survival have not yet been evaluated (4). In this study, we assessed the molecular and functional consequences of TNF-alpha treatment in Caco-2 colon adenocarcinoma cells and determined the radiation sensitivity of these cells in order to apply TNF-alpha as a therapeutic agent for cancer. Caco-2 cells were found to be highly resistant to TNF-alpha-induced cell death. Although it was not linked to morphological changes, molecular alterations in E-cadherin and claudin-4 were observed. However, combined treatment with TNF-alpha and 2 Gy of γ-irradiation significantly reduced the survival of Caco-2 cells and induced molecular changes in bcl-xl and PARP after the activation of JNKs. These results indicate that TNF-alpha may potentially be applied in combination with radiotherapy for the treatment of human colon cancer.
E-cadherin is an important adhesion molecule in cell-cell junctions, and it has been previously reported that the downregulation of E-cadherin is correlated with tumor progression in many epithelial-derived tumors (16). In cases of hereditary diffused gastric cancer (HDGC), the deregulation of E-cadherin renders cells resistant to apoptosis (18); bcl-2 overexpression reduces E-cadherin-mediated junctions and causes unregulated growth in MCF-7 cells(19). Additionally, E-cadherin is involved in the pro-apoptotic process via the regulation of the caveolin-1-mediated downregulation of survivin, an apoptosis inhibitor in HT 29 and B16-F10 melanomas (20). Therefore, the downregulation of E-cadherin noted in TNF-alpha-treated Caco-2 cells was probably a favorable condition for cell survival rather than cell death. Additionally, alterations in junctional proteins render cells unstable and ready to respond to secondary apoptotic stimuli. Two Gy of irradiation coupled with pre-treatment of TNF-alpha altered Caco-2 cells rendering them to be susceptible to irradiation-induced cell death.
Bcl-xl is a member of the anti-apoptotic bcl-2 family and promotes cell survival through the regulation of the electrical and osmotic homeostasis of the mitochondria in response to a variety of stresses (21). Particularly in Caco-2 cells, the forced expression of the antisense oligonucleotides for bcl-xl enhances apoptosis under irradiated conditions (17). We could anticipate that high levels of bcl-xl might prove critical for the regulation of cell survival. Unexpectedly, both TNF-alpha and 2 Gy of γ-irradiation downregulate bcl-xl expression levels in Caco-2 cells. However, these effects were not linked to the reduction of the surviving fraction of Caco-2 cells as the deregulation of E-cadherin maintained the survival characteristics of cells under each condition. The effect of cell death was observed only in cases when the combined treatment of TNF-alpha and 2 Gy of γ-irradiation were applied which resulted in a synergistic downregulation of bcl-xl. PARP cleavage also increased in a synergistic fashion as the result of the combined treatments of TNF-alpha and γ-irradiation.
JNKs are known as stress-activated signaling molecules and induce cell death under environmental stress conditions (22). The activation of JNKs was detected only in the combined treatments of TNF-alpha and 2 Gy of γ-irradiation. This finding correlated well with the enhanced cell death in Caco-2 cells via TNF-alpha and 2 Gy of γ-irradiation. In terms of time, the activation of JNKs occurs earlier than other molecular alterations. Therefore, we speculate that single treatment with TNF-alpha is not sufficient for the induction of Caco-2 cell death. It is possible that alterations in E-cadherin and claudin-4 junctional proteins may suppress JNK activation and cell death in Caco-2 cells. However, junctional modulations occurring as the consequence of TNF-alpha treatment may promote cell death in response to other additional cell death stimuli, in this case, 2 Gy of γ-irradiation. The radiosensitizing roles for TNF-alpha were only effective for 2 Gy of γ-irradiation, a relatively low dose, compared to 4 and 8 Gy. At high doses of γ-irradiation, 4 Gy and 8 Gy, enhanced cell death via TNF-alpha pre-treatment was impaired. This could be caused by excessive death signals from irradiation-induced DNA damages. We could not discriminate the additive effects of irradiation from the TNF-alpha-induced effects of the junctional modulations and apoptotic signaling molecules.
The downregulation of E-cadherin and claudin-4 induced by TNF-alpha treatment did not sufficiently activate JNKs and cell death, and 2 Gy of γ-irradiation alone also proved insufficient for the induction of cell death. Only the combination of 2 Gy of γ-irradiation with TNF-alpha pre-treatment was shown to activate JNKs and enhance cell death. In conclusion, the results of this study indicate that TNF-alpha may potentially be applied in combination with a low dose of radiotherapy to treat human colon cancer.
ConclusionCaco-2 cells were determined to be highly resistant to TNF-alpha-induced cell death although molecular alterations of adhesion proteins were observed. However, combined treatment with TNF-alpha and 2 Gy of γ-irradiation effectively reduced the survival of Caco-2 cells and induced molecular changes in bcl-xl and PARP following the activation of JNKs. These results suggest that TNF-alpha may be potentially applied in combination with radiotherapy at a low dose, 2 Gy, as a treatment for human colon cancer.
NotesThis work was supported by a Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund, KRF-2006-003-C00220), Nuclear Research Development Program of the Korea Science and Engineering Foundation (KOSEF M2070600005-08B0600-00510), and National Nuclear Research & Development Program of the Korean Ministry of Science and Technology (2007-00324). References1. Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL. Anti-TNF-alpha therapies: the next generation. Nat Rev Drug Discov. 2003;2:736–746. PMID: 12951580
2. Younes A, Aggarwall BB. Clinical implications of the tumor necrosis factor family in benign and malignant hematologic disorders. Cancer. 2003;98:458–467. PMID: 12879461
3. Younes A, Kadin ME. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy. J Clin Oncol. 2003;21:3526–3534. PMID: 12972530
4. Jones SA, Butler RN, Sanderson IR, Wilson JW. The effect of specific caspase inhibitors on TNF-alpha and butyrate-induced apoptosis of intestinal epithelial cells. Exp Cell Res. 2004;292:29–39. PMID: 14720504
5. Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. PMID: 12887920
6. Kimura K, Bowen C, Spiegel S, Gelmann EP. Tumor necrosis factor-alpha sensitizes prostate cancer cells to gamma-irradiation-induced apoptosis. Cancer Res. 1999;59:1606–1614. PMID: 10197636
7. Schmelz K, Wieder T, Tamm I, Muller A, Essmann F, Geilen CC, et al. Tumor necrosis factor alpha sensitizes malignant cells to chemotherapeutic drugs via the mitochondrial apoptosis pathway independently of caspase-8 and NF-kappaB. Oncogene. 2004;23:6743–6759. PMID: 15273737
8. Anderson GM, Nakada MT, DeWitte M. Tumor necrosis factor-alpha in the pathogenesis and treatment of cancer. Curr Opin Pharmacol. 2004;4:314–320. PMID: 15251122
9. Li Q, Zhang Q, Wang M, Zhao S, Ma J, Luo N, et al. Interferon gamma and tumor necrosis factor-alpha disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol. 2008;126:67–80. PMID: 17964857
10. Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, et al. Tumor necrosis factor-alpha (TNF-alpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137–146. PMID: 9841910
11. Suk K, Chang I, Kim YH, Kim S, Kim JY, Kim H, et al. Interferon gamma (IFNgamma) and tumor necrosis factor alpha synergism in ME-180 cervical cancer cell apoptosis and necrosis. IFNgamma inhibits cytoprotective NF-kappa B through STAT1/IRF-1 pathways. J Biol Chem. 2001;276:13153–13159. PMID: 11278357
12. Angelini DJ, Hyun SW, Grigoryev DN, Garg P, Gong P, Singh IS, et al. TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1232–L1245. PMID: 16891393
13. Bove K, Neumann P, Gertzberg N, Johnson A. Role of ecNOS-derived NO in mediating TNF-induced endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;280:L914–L922. PMID: 11290515
14. Eggermont AM, De Wilt JH, Ten Hagen TL. Current uses of isolated limb perfusion in the clinic and a model system for new strategies. Lancet Oncol. 2003;4:429–437. PMID: 12850194
15. Folli S, Pelegrin A, Chalandon Y, Yao X, Buchegger F, Lienard D, et al. Tumor-necrosis factor can enhance radio-antibody uptake in human colon carcinoma xenografts by increasing vascular permeability. Int J Cancer. 1993;53:829–836. PMID: 8449608
16. Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. PMID: 17854762
17. Wacheck V, Selzer E, Gunsberg P, Lucas T, Meyer H, Thallinger C, et al. Bcl-x(L) antisense oligonucleotides radiosensitise colon cancer cells. Br J Cancer. 2003;89:1352–1357. PMID: 14520471
18. Ferreira P, Oliveira MJ, Beraldi E, Mateus AR, Nakajima T, Gleave M, et al. Loss of functional E-cadherin renders cells more resistant to the apoptotic agent taxol in vitro. Exp Cell Res. 2005;310:99–104. PMID: 16112667
19. Li L, Backer J, Wong AS, Schwanke EL, Stewart BG, Pasdar M. Bcl-2 expression decreases cadherin-mediated cell-cell adhesion. J Cell Sci. 2003;116:3687–3700. PMID: 12890751
20. Torres VA, Tapia JC, Rodriguez DA, Lladser A, Arredondo C, Leyton L, et al. E-cadherin is required for caveolin-1-mediated down-regulation of the inhibitor of apoptosis protein survivin via reduced beta-catenin-Tcf/Lef-dependent transcription. Mol Cell Biol. 2007;27:7703–7717. PMID: 17785436
21. Gottlieb E, Vander Heiden MG, Thompson CB. Bcl-x (L) prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol Cell Biol. 2000;20:5680–5689. PMID: 10891504
22. Vivo C, Liu W, Broaddus VC. c-Jun N-terminal kinase contributes to apoptotic synergy induced by tumor necrosis factor-related apoptosis-inducing ligand plus DNA damage in chemoresistant, p53 inactive mesothelioma cells. J Biol Chem. 2003;278:25461–25467. PMID: 12707267
Fig. 1Caco-2 cells are resistant to cell death induced by TNF-alpha treatment. (A) Cell viability was not altered by TNF-alpha treatment. Cytotoxicity assays were conducted on TNF-alpha-treated Caco-2 cells at 10 and 100 ng/mL concentrations. Caco-2 cells were seeded at 5×103 cells in 96-well plates and permitted to attach to plates for 2 days, after which TNF-alpha was added. After 3 days of incubation, 10 µL of color reagent was added to each well, and the cells were incubated for 1 h. The mean absorbance at 450 nm in each set of samples was measured. Shown are the mean percentages of cell survival ± S.E. of three independent experiments. (B) Downregulation of E-cadherin was noted in the TNF-alpha (10 ng/mL)-treated Caco-2 cells, but not claudin-4. E-cadherin, and claudin-4 expression levels were assessed via Western blot. Total cell lysates were prepared. (C) Downregulation of E-cadherin and altered claudin-4 translocation were noted in the TNF-alpha (10 ng/mL)-treated Caco-2 cells. Immunofluorescence staining for E-cadherin and claudin-4 was conducted on Caco-2 cells cultured on cover slips. The cells were fixed in 4% paraformaldehyde, permeabilized for 5 min with 0.1% Triton X-100, and antibodies were added at a dilution recommended by the manufacturer. Laser scanning confocal microscopy was conducted with a MRC-1024/ES confocal microscope. 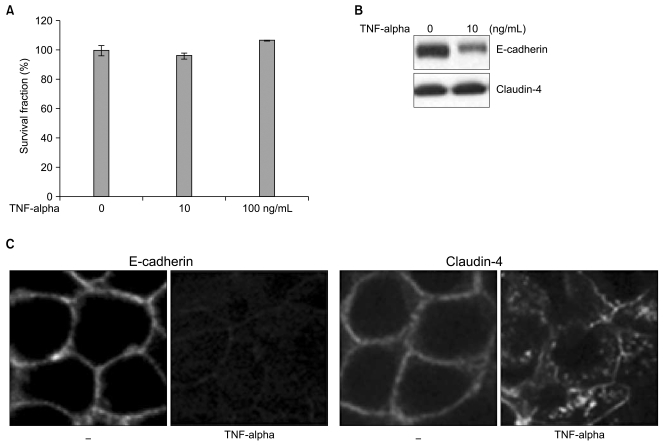
Fig. 2Reduced cell survival of Caco-2 via combined treatment with TNF-alpha and 2 Gy of γ-irradiation. (A) Clonogenic assays were conducted to determine cell survival under the following experimental conditions: cells were treated separately with TNF-alpha (10 ng/mL) and γ-irradiation (2 Gy) or cells were pre-treated with TNF-alpha for 16 hr and then exposed to 2 Gy of γ-irradiation (combined treatment). After 10 days of incubation, the colonies were stained with crystal violet and the number of colonies was counted. The percent of cell survival was obtained by counting the surviving colonies. Shown are the mean percentages of cell survival±S.E. of three independent experiments. (B) The surviving fractions of TNF-alpha treatment for control were obtained to represent relative changes of surviving fractions under various doses of γ-irradiation conditions, 2 Gy, 4 Gy, and 8 Gy following TNF-alpha treatment. Only 2 Gy of γ-irradiation exposure to pre-treated TNF-alpha (10 ng/mL) cells exhibited reduction of cell survival. Shown are the surviving fraction of TNF-alpha treatment divided by the control surviving fraction, indicated below as a table, the mean percentages of cell survival±S.E. of three independent experiments. 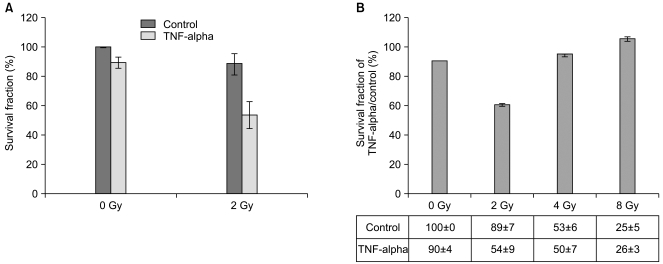
Fig. 3TNF-alpha activates pro-apoptotic signals and JNKs. (A) The synergistic alterations of apoptotic signaling molecules were noted in the combined treatment of TNF-alpha with 2 Gy of γ-irradiation. Increased cleavage of PARP and downregulation of bcl-xl were assessed in cells treated with TNF-alpha in combination with 2 Gy of γ-irradiation. Total cell lysates were prepared and α-tubulin was utilized as a loading control. (B) TNF-alpha induces JNK activation in response to 2 Gy of γ-irradiation. The phosphorylation of JNK-1, 2, and 3 was detected after the combined treatment of TNF-alpha and 2 Gy of γ-irradiation in Caco-2 cells. The cells were pre-treated with TNF-alpha (10 ng/mL) for 16 hr prior to γ-irradiation and harvested after 6 hrs of exposure. JNK-1, 2, and 3 phosphorylation were only observed in the combined treatment of TNF-alpha and 2 Gy of γ-irradiation. Total cell lysates were prepared and α-tubulin was utilized as the loading control. 
|
|
||||||||||||||||||||||||||||||||||||||