Introduction
For patients suspicious for prostate cancer by screening surveillance, the final diagnosis has been made by transrectal ultrasound-guided biopsies of 6~14 cores according to the physician's preference. After the introduction of serum prostate specific antigen (PSA) into the clinical screening strategy for prostate cancer, the stage at pathologic confirmation has decreased. As a result, most of the diseases discovered have been localized cancers (1). For these localized tumors, radical prostatectomy (RP) is the standard established treatment option, and the goals of RP in this stage are cancer cure, minimization of complications and perioperative suffering, and preservation of urinary continence and erectile function (2).
To achieve these goals, urologists typically recommend that RP be performed at least 4 to 6 weeks after biopsy in spite of the patient's anxiety for early operation (3). This delay presumably allows inflammatory adhesions and hematomas to resolve, so that the anatomic relationships between the prostate and its surrounding structures return to a nearly normal state before surgery. In terms of the number of biopsy cores, an increase over the traditional six cores is required to improve tumor detection rate. However, this can potentially aggravate the technical difficulty of RP by inducing inflammation and bleeding.
While there is no distinct surgical modality to resolve these dilemmas related to prostate biopsy, robotic assisted laparoscopic radical prostatectomy (RALP) can be a surgical approach that maintains the goals of RP. RALP provides several benefits in precise dissection through improved instrument control with articulating tips, 3-dimensional vision, and a magnified surgical field (4). If this new modality enables safe and feasible operations regardless of biopsy core number or the interval from biopsy to operation, it will provide another advantage in biopsy strategy for both patient and physician, widening surgical indications of RP. To evaluate the possible role of RALP, we prospectively investigated the effect of biopsy related prostate status on the clinicopathologic outcome of RALP in our institution.
Materials and Methods
1 Patient and data analysis
From July 2007 to April 2009, 72 consecutive patients with clinically localized or locally advanced prostate cancer underwent RALP by a single surgeon (J. Cheon) after an initial learning period of 30 cases (5). For these patients, peri-operative variables including prostate biopsy core number and interval to operation were collected prospectively and analyzed. All patients had biopsy proven adenocarcinoma of the prostate, which were staged according to the 1992 American Joint Committee on Cancer (AJCC) classification (6).
All patients underwent digital rectal examination, transrectal ultrasonography, whole body bone scan, and abdominal CT or MRI to determine preoperative stage. To investigate intra-operative outcomes, the operative time (including console time and set up time), and estimated blood loss were surveyed. In this series, the total operative time was defined as the time from initial incision to skin closure. Post-operatively, information on the hospital stay, catheterization period, pathologic result (including positive surgical margin), and development of complications were collected.
The impact of biopsy core number and interval to RP on operative time and EBL were evaluated initially by linear regression. To evaluate the influences of the previously established recommendations of a 4 week interval and a biopsy core number of 10, the patients were sub classified into two groups according to the interval from biopsy to surgery using a cutoff point of 4 weeks, and according to the total number of biopsy cores with a cutoff point of 10 cores. Among these groups, the differences in mean operation time, EBL, transfusion rate, positive-margin rate, bladder neck reconstruction rate, and nerve-sparing rate were evaluated by univariate analysis (Mann-Whitney U test, Pearson's chi-square test) and univariate Logistic regression analysis. The statistical analysis program SPSS ver. 12.0 (SPSS, Chicago, IL) was used, and a p-value below 0.05 was considered statistically significant.
2 Surgical procedures
Surgery was performed by standard transperitoneal approach and interfacial technique, as reported by Patel et al (7). The dorsal vein complex was ligated with a 1-0 Monocryl stitch (Ethicon INC, Cornelia, GA), and a second 1-0 Monocryl stitch was then placed through the periurethral tissue and periosteum of the posterior pubic symphysis, for anterior reconstruction. To optimize nerve sparing, all prostatic pedicles were clipped with Hem-o-lok polymer ligating clips (Weck Closure Systems, Research Triangle Park, NC), then sharply divided. For the patient with planned nerve preservation, a neurovascular bundle (NVB) sparing procedure was performed with the method originally described by Patel (7). After the NVB was released from the posterolateral aspect of the prostate, the dissection was extended distally towards the prostatic apex. The attachment of the NVB and prostate was then dissected in a retrograde manner, and then divided after clipping. The urethrovesical anastomosis was performed in a running fashion using a double-arm 3-0 Monocryl suture.
Results
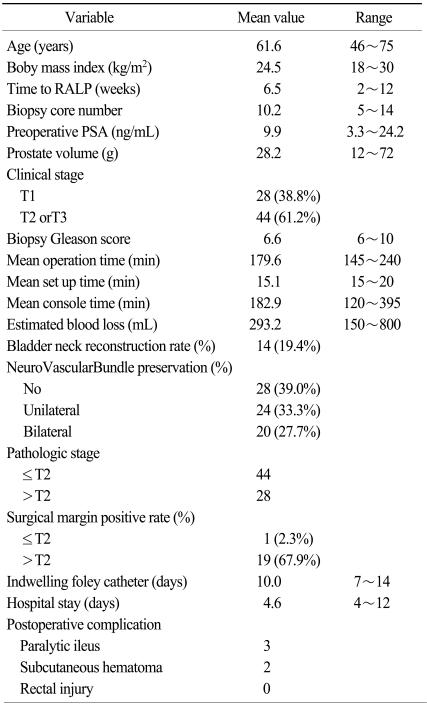
RALP patient characteristics are summarized in Table 1. Mean age (range) was 61.6 (46~75) years old and body mass index was 24.5 (18~30) kg/m2. The mean interval from biopsy to RALP was 6.5 (2~12) weeks and the mean biopsy core number was 10.2 (5~14). Mean operative time was 218.9 (170~350) minutes and mean EBL (estimated blood loss) was 293.2 (150~800) mL. Bladder neck reconstruction rate was 19.4% and nerve-sparing rate was 61.0%. Lengths of hospital stay and foley catheter indwelling time were 17.0 and 8.6 days, respectively. A positive surgical margin was present in only one of 44 patients with localized (≤pT2) tumors (2.3%). In this patient, RALP was performed 6 weeks after a 10-core prostate biopsy.
The complication rate was 6.9%. No patient had major complications such as open conversion, rectal injury, major vessel injury, or thromboembolic diseases. Three patients had postoperative ileus (two transient, one prolonged), and two patients had trocar insertion-related subcutaneous hematomas. All of these minor complications were resolved following conservative management.
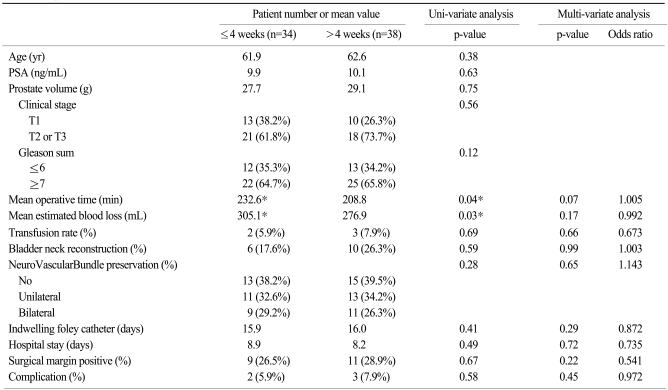
After subdividing the patients into two groups, operative outcomes were stratified by time interval to RALP and summarized in Table 2. The resulting two groups had similar characteristics in age, preoperative PSA, prostate volume, tumor stage, and gleason score. The mean intervals from biopsy to RP for the groups that underwent RP within 4 weeks (n=34) and after 4 weeks (n=38) were 3.2 (2~4) weeks and 8.3 (5~13) weeks, respectively. Mean operative time for RALPs performed within 4 weeks was longer (232.6 min vs 208.8 min) than those performed after 4 weeks. EBL was greater for the RALPs performed within 4 weeks (305.1 mL vs 276.9 mL), though more transfusions were not needed. These differences were not reproduced in a multi-variate analysis. Positive-margin rate, complication rate, bladder neck reconstruction rate, nerve-sparing rate, length of hospital stay, and indwelling foley catheter time showed no difference regardless of interval to RP.
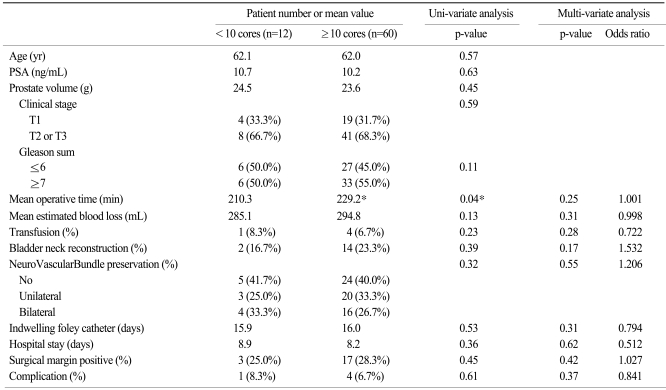
Table 3 reveals operative differences according to biopsy core numbers. These two groups were also similar in age, preoperative PSA, prostate volume, tumor stage, and gleason score. The mean core number less than 10 (n=10) was 6.3 (5~9) and the mean core number greater than 10 (n=62) was 11.2 (10~14). In cases of extended biopsy (more than 10 cores obtained), operative time was longer (229.2 min vs 210.3 min), but no clinical significance was observed on multi-variate analysis. Other clinical parameters were similar. Thus, all the differences showed no statistical significance on univariate analysis, and there were no actual increases in operative time or EBL in cases with early operation or extended core biopsy.
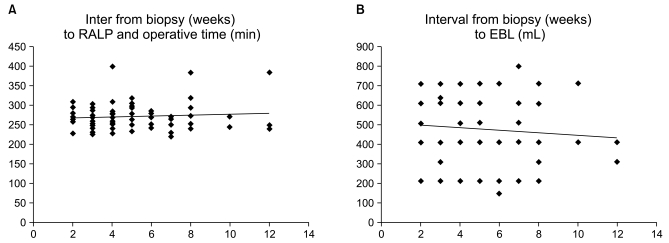
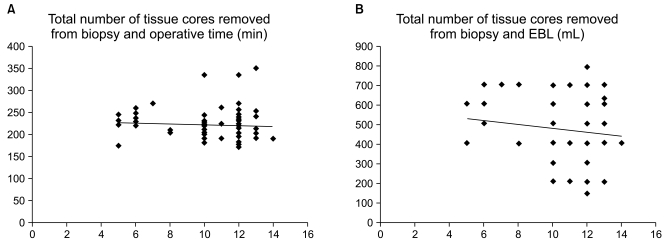
Linear correlation analysis was used to compare the interval from biopsy to RALP with both operative time and EBL, and there was no correlation (correlation coefficient 0.24, p=0.09 and 0.26, p=0.14, Fig. 1). Similarly, the number of core specimens also showed no relationship with either operative time or EBL (correlation coefficient 0.10, p=0.35 and 0.09, p=0.53, Fig. 2).
Discussion
Scheduling for radical prostatectomy (RP) after biopsy remains a problematic decision. Many urologists contend that performing RP soon after a biopsy increases surgical difficulty and complication rates. Urologists have typically recommended that radical prostatectomy be performed at least 4 to 6 weeks after biopsy, presumably to allow for resolution of biopsy-induced inflammation and bleeding. But the basis of this conception is limited. Imaging studies by endorectal MRI after prostate biopsy suggest that changes can last up to 21 days (8,9). The serum PSA level generally returns to baseline 4 weeks after biopsy, and is considered a sign of complete resolution of inflammation (10). These findings support the concept of waiting a minimum of 4 weeks before conducting an RP, and this was empirically used as a guideline period. However, a recent report that retrospectively reviewed the charts of 169 patient who had undergone retropubic RP showed that the interval to RP is not associated with operative time, length of hospital stay, or complications (11). The only variable that approached statistical significance was EBL. Another study of 2,996 patients did not find a significant association of shorter intervals with EBL, operative time, positive surgical margins, postoperative erectile dysfunction, or urinary incontinence (12). All patients in the present analysis had retropubic RP, and these findings are applicable to RP performed via the robotic or laparoscopic approach (13,14). In addition, because many treatment options are available for clinically localized prostate cancer, significant periods are often required before finalizing the treatment choice decision. In addition, most large studies have suggested that a delay between biopsy and RP does not compromise postoperative disease-free progression (15,16). Operative difficulties or bleeding may be ascribable to local unresolved inflammation, or greater difficulty in dissecting between tissue planes.
Our study revealed more information on this issue. In early RALP done 2~4 weeks after biopsy, the operative time and EBL were arithmetically increased compared to that in the delayed RALP group, but these differences did not reach statistical significance. In contrast to most reports that have been published, about 100 patients underwent RALP in this study. This implies that surgical feasibility and safety for early RP could be obtained during initial learning period of the RALP, and this may be another advantage of this novel modality.
So the current recommendation of waiting 4-weeks or more from biopsy to RP might not be necessary in all patients to guarantee a reduction in operative complications, and early RP from prostate biopsy does not appear to induce more technical difficulty in our study. Because urologists often encounter impetuous patients that choose RP and request a surgical date as soon as possible, these findings may provide reassurance to urologists and patients that choose RP relatively soon after biopsy.
Biopsy core number usually has focused on the cancer detection rate rather than surgical influences. Most urologists agree that the sextant biopsy is inadequate and initial prostate biopsy strategies should include at least 10~14 cores. To improve prostate cancer detection, more than 10 core extended biopsy protocols were developed. Presti et al. prospectively evaluated 10-core biopsy in 483 patients and found that sextant biopsy alone was inadequate, as 20% of cancers were missed (17). Six-core biopsy detected only 71% of cancers found by 12-core biopsy (18). Short term complications from biopsy were similar. Berger et al. compared complication rates between 6, 10, and 15-core biopsy protocols (19). Their study revealed no difference in major complications (fever, prostatitis, epididymitis, or urinary retention), although there was an increased number of patients with hematospermia after 10-core and 15-core biopsies. The mean number of tissue cores obtained at diagnostic prostate biopsy increased from 6.9 in 1995 to 10.2 in 2004. The trend toward an increasing number of removed cores may have contributed indirectly to improved outcomes after RP by decreasing the percentage of positive cores and being associated with recurrence-free survival after radical prostatectomy (20). But the effect of an increased biopsy core number compared to conventional sextant biopsies on surgical outcome (including operative time and blood loss) remains unclear. The increased number of biopsy cores did not influence the operative parameters in our study except for a comparatively longer operative time. Extended biopsy includes conventional sextant biopsy cores - at the base, mid-gland, and apex of the prostate - and additional cores laterally directed at the base, mid-lobar and apex. These extra cores have a more superficial location, so we can better predict adhesive tissue effect and inflammation from biopsy. The effect of an increased biopsy core number on operation was negligible in our study.
This report on biopsy core number and time from biopsy to operation in RALP has limitations. There is lack of analysis in functional outcome regarding potency and continence due to a relatively short follow up period. Also, while the positive surgical margin was similar in each group, information on long term oncologic safety in terms of biologic recurrence is still needed. Furthermore, many biopsies were conducted in different hospitals with different methods. In spite of these limitations, this study on biopsy core number and the interval between prostate biopsy to RALP gives positive support to patients who are anxious for quick operations and more extensive biopsies to cure their disease. According to our results, clinicians could be more free to select the time of RALP and less distressed about prostate condition from increased biopsy core numbers in RALP.
Conclusion
In our data, advancing the time of RALP to 2~4 weeks after biopsy did not impact surgical and pathologic outcomes compared to the conventional delay of over 4 weeks. Similarly, extension of biopsy core number to 14 cores did not influence the short term result of RALP. These together imply that RALP can be conducted feasibly regardless of biopsy related prostate status. However, longer and larger studies would be needed to support this concept shift, particularly in terms of functional and biological recurrence.