Introduction
Prostate cancer is the most common cancer in men, with approximately 186,320 new cases and 28,660 deaths occurring annually in the USA (1). In Korea, as dietary habits change, detection methods improve, and the size of the elderly population increases, cases of prostate cancer are increasing at a rapid pace. In fact, the estimated number of new cases in 2005 was 3,487, representing a 243% increase over the time period from 1999 (n=1,497) (2).
Treatment of metastatic prostate cancer is palliative. Androgen ablation therapy is the cornerstone of first-line therapy, producing a rapid improvement in bone pain and soft tissue metastasis, with a prompt decrease in prostate-specific antigen (PSA) levels. However, the effect of hormone therapy is transient with a median response duration of 12~33 months (3), with the disease eventually gaining resistant to this treatment, developing hormone-refractory prostate cancer (HRPC). The vast majority of prostate cancer-related deaths occur as a consequence of progressive HRPC.
Two large trials in the late 1990s demonstrated that the combination of mitoxantrone and corticosteroids could provide pain-relief and decrease PSA levels, with improved QOL compared with corticosteroid alone. However, with this treatment regiment there was no improvement in survival; time to symptomatic progression is of the order of 3.7 to 4.5 months (4,5). Since 2004, treatment of HRPC has considerably evolved with the reporting of 2 landmark studies, TAX327 and SWOG 99-16, which revealed that docetaxel-based chemotherapy not only improved the quality of life and PSA response, but prolonged survival of patients with HRPC, as well (6,7).
Based on this background, new therapeutic approaches such as new biologics and cytotoxic combinations are being pursued. However, cancer researchers have given little attention to the potential application of this treatment type in Korean patients with metastatic HRPC. In the present study, our goal was to determine the efficacy and safety of docetaxel plus prednisolone combination chemotherapy in the treatment of metastatic HRPC in the clinical practice setting.
Materials and Methods
1. Patients
Between October 2003 and April 2008, 98 patients with HRPC were treated with docetaxel-based chemotherapy at the Asan Medical Center. All patients who fulfilled the following eligibility criteria were enrolled into the study: (1) histologic diagnosis of adenocarcinoma of the prostate with clinical or radiological evidence of metastatic disease; (2) documented disease progression during hormonal therapy defined by Prostate Cancer Clinical Trials Working Group (PCWG) criteria (Table 1) (8); (3) a minimum of 4 weeks or 6 weeks lapse between the withdrawal of flutamide or bicalutamide, respectively, to avoid the possibility of antiandrogen withdrawal phenomenon; (4) Eastern Cooperative Oncology Group performance status of 2 or better; (5) no prior treatment with mitoxantrone or radioisotope; (6) no history of another cancer within the preceding fiver years; (7) no evidence of central nervous system metastasis; (8) no serious or uncontrolled concomitant medical illnesses; and (9) adequate bone marrow and organ function. Patients were ineligible if they had received weekly docetaxel treatment or docetaxel-estramustine combination chemotherapy. The institutional review board of the Asan Medical Center granted permission for this retrospective study.
2. Treatment protocol and dose adjustments
This was a retrospective cohort study that examined the medical records of patients with HRPC, who had been treated with docetaxel-based chemotherapy. Patients received docetaxel 75 mg/m2 administered as 1-hour intravenous infusion on day 1, followed by one dose every 3 weeks with 5 mg oral prednisolone twice daily starting on day 1 and continuing throughout treatment (7). Premedication with dexamethasone for the prevention of fluid retention was administered. Prophylactic administration of G-CSF was not performed as this is not reimbursed by the Korean National Health Insurance policy. There was no preset maximal number of chemotherapy cycles and the treatment course was repeated until we observed disease progression, unacceptable toxicities, clinically significant concomitant illnesses, or patient refusal to continue treatment.
3. Safety and efficacy assessment
Prior to treatment, all patients had a detailed medical history, physical examinations, and baseline laboratory measurements performed. Pretreatment tumor status was evaluated using computed tomography or magnetic resonance imaging, and bone scans when necessary. Patients were seen on day 1 of every treatment cycle for a brief history, physical examination, and assessment of adverse events, complete blood count, renal and liver function testing, and PSA levels. Toxicities were re-evaluated according to the National Cancer Institute-Common Terminology Criteria of Adverse Events (NCI-CTCAE), version 3.0. There was no preset interval for tumor response imaging evaluation, but reevaluations occurred after the 6th cycle or after clinical signs of disease progression.
4. Statistical analysis
The primary endpoint was PSA response and the secondary endpoints were overall survival (OS), safety, and time to PSA progression. Data collected included pretreatment disease characteristics, baseline biochemical parameters, prior therapies, first date of treatment. The 12-week and maximal PSA declines to treatment, date of PSA progression, date of symptomatic deterioration or tumor progression, and date of death or last follow-up. Kaplan-Meier estimates and the log-rank test were used to analyze time-event variables. Patients were considered assessable if they received at least 2 cycles of chemotherapy and at least 2 follow-up PSA assessments. Patients were considered assessable if they received fewer than the predefined number of chemotherapy cycles because of rapid tumor progression. All tests were 2 sided and a p-value<0.05 was considered statistically significant. SPSS for Windows version 13.0 (SPSS Inc., Chicago, IL) was used for statistical analyses.
Results
1. Patient characteristics
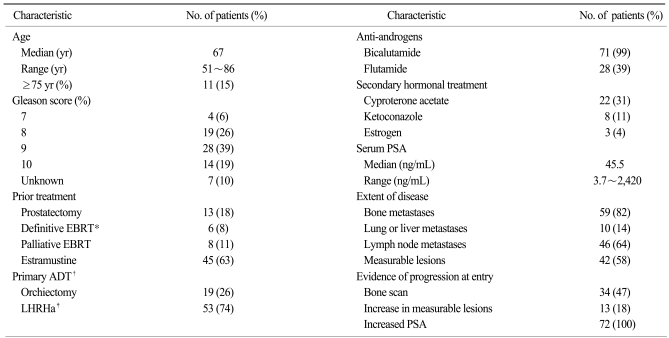
Seventy-two of 98 HRPC patients who received docetaxel chemotherapy fulfilled the eligibility criteria for enrollment into the study. The patient demographics and disease characteristics are summarized in Table 2. Twenty-six patients were excluded for the following reasons: concomitant advanced gastric cancer (n=1); no evidence of distant metastasis (n=3); PSA value less than 2 ng/mL (n=2); serum creatinine level more than 2 mg/dL (n=1); prior exposure to radioisotope (n=1); Poor performance status (n=1); prior mitoxantrone plus prednisolone treatment (n=11); and concomitant administration of estramustine with weekly docetaxel (n=10). Three patients had multiple reasons for exclusion from the study.
2. Drug delivery and safety
At the time of analysis, 70 patients were taken off the treatment and 2 patients remain on chemotherapy. A total of 405 cycles of treatment were administered to 72 patients (median 6 cycles; range, 1~20 per patients). The frequencies of treatment-related hematological and nonhematological adverse events are shown in Table 3. Events of hematologic grade 3~4 toxicity included neutropenia (17%), leucopenia (15%), febrile neutropenia (13%), and anemia (6%). Asthenia (6%) was the most common non-hematologic grade 3 or worse event. There were no treatment-related deaths directly attributable to docetaxel chemotherapy. Docetaxel dose reduction was required in 2 of the 64 patients (3%) who received at least two cycles of treatment. The median docetaxel dose-intensity was 24.4 mg/m2/week (range, 17.5~25.6) with a relative dose intensity of 98%. When grade 3 or worse toxicities developed, in particular febrile neutropenia, the majority of the patients were withdrawn from the treatment. Considering this fact, the proportion of patients who required dose reduction may have been underestimated. Disease progression was the most common reason for discontinuation of chemotherapy (38 out of 70, 54%), followed by treatment-related adverse events (13%), and patient refusal of treatment (9%). Forty-four patients (63%) received subsequent cytotoxic chemotherapy with mitoxantrone plus prednisolone (31 patients, 44%), estramustine (23 patients, 33%), or cyclophosphamide-based combination chemotherapy (7 patients, 10%). Salvage hormonal therapy was also given to 40% of patients after treatment failure.
3. Efficacy
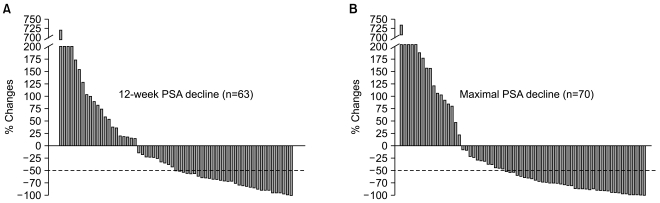
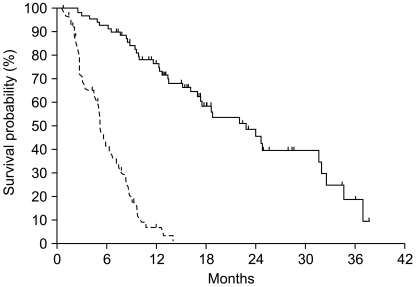
Post-chemotherapy 12-week and maximal PSA decline in response to treatment is shown in Fig. 1. A PSA response (confirmed PSA decline ≥50%) was seen in 32 of 63 evaluable patients (51%) at 12 weeks and a maximal PSA decline of 50% or more was seen in 42 of 70 evaluable patients (59%). Among 42 patients with measurable disease, tumor response was reevaluated in 13 patients. Four patients achieved a partial response and 5 patients maintained stable disease. With a median follow-up duration of 23.1 months using reverse Kaplan-Meier methods (95% CI, 16.7~29.5), the median time to PSA progression was 5.1 months (95% CI, 4.48~5.75) and median OS was 22.8 months (95% CI, 16.6~29.1) (Fig. 2).
Discussion
The results of the TAX 327 and SWOG 99-16 studies demonstrated a survival benefit of docetaxel-based chemotherapy in patients with metastatic HRPC (6,7,9). The regimen of 3 weekly docetaxel (75 mg/m2) plus low-dose prednisolone is widely considered to be the treatment of choice for symptomatic, metastatic HRPC.
However, these studies mainly included patients from Western countries. The reports on the efficacy and safety of docetaxel-based chemotherapy in patients with Asian ethnicity are very limited; only one Japanese phase II study on reduced dose of docetaxel and one Korean retrospective study including inhomogeneous patients with a short follow-up duration (10 months) could be found in the English medical literature (10,11). Docetaxel metabolism has been reported to be affected by the ethnicity (12,13), by castration status (14), and by age (15). Inferring the toxicity and efficacy from other studies that enrolled patients with Western ethnicity, non-castrate status, and of younger age may result in erroneous conclusions, and data from Korean patients is urgently required. To our knowledge, this study is the largest investigation on the efficacy and safety of docetaxel chemotherapy in Korean men with HRPC.
In terms of efficacy, the PSA response of 51% and median OS of 22.8 months are comparable to or even better than those from TAX 327 study, which revealed the PSA response rate of 45% and median OS of 19.2 months (7,16). Although the definition is not exactly the same, the time to PSA progression (5.8 months) in the current study is also comparable to the time to progression reported in SWOG 99-16 study (6.3 months) (6). The clinico-pathologic characteristics of the current study were quite similar to those of the TAX327 and SWOG 99-16 studies, with the exception of the fact that more patients with a Gleason score ≥8, one of the known poor prognostic factors for OS, were included in this study.
Our results showed that the adverse events were acceptable and within predictable range. However, febrile neutropenia occurred much more frequently (13%, 95% CI, 5~20%) than those of phase III studies or systemic review incorporating Western patients (3~6%) (6,7,17). These serious complications developed without G-CSF prophylaxis or antimicrobial prophylaxis. Fortunately and in general, the complications were managed successfully with antibiotics and G-CSF. Considering the fact that febrile neutropenia also developed in 16% of Japanese patients with HRPC treated with lower docetaxel (70 mg/every 3 weeks) and 18% of Korean patients with other solid malignancy receiving docetaxel (75 mg/m2 every 3 weeks) (10,18), patient ethnicity could be a major contributing factor to docetaxel-associated febrile neutropenia. Caution should be exercised when treating patients of Asian ethnicity with this regimen, particularly in regard to the development of febrile neutropenia, in patients of older age, or with poor performance status. Sequential dose escalation based on toxicities observed during the first cycle of chemotherapy or prophylactic use of antibiotics or G-CSF might be a reasonable approach in these cases (19,20).
Docetaxel plus prednisolone chemotherapy is now the standard of care for men with metastatic HRPC. However, the benefit of docetaxel plus prednisolone chemotherapy is limited (21): the efficacy of the drug is limited in one-third to one half of patients; a median time to PSA progression was on the order of 5~8 months (6); the absolute survival benefit is only 2.4 months (6,7); and it accompany grade 3 or worse toxicities in up to 45% of patients (6,7). There is a clear need for therapies that improve outcomes, and the results of ongoing phase III trials incorporating abirateron (22), atrasentan (23), zibotentan, or bevacizumab are eagerly needed (24).
There were several limitations in this study. First is the inherent selection bias and potential data imperfection of the retrospective study design. The second is the lack of preset radiologic reassessment even in patients with measurable lesions, which made radiologic response evaluation difficult. Finally, patient-reported outcomes, such as quality of life, could not be determined.