AbstractPurposeThis study evaluated the efficacy of extended field irradiation (EFI) in patients with locally advanced cervical cancer without para-aortic nodal involvement.
Materials and MethodsA total of 203 patients with locally advanced cervical cancer (International Federation of Gynecology and Obstetrics [FIGO] stage, IB2-IIIB) treated with radiotherapy at Keimyung University Dongsan Medical Center from 1996 to 2010 were retrospectively analyzed. The median patient age was 59 years (range, 29 to 83 years). None of the patients had para-aortic node metastases. Of the 203 patients, 88 underwent EFI and 115 underwent irradiation of the pelvis only. Concurrent chemoradiotherapy (CCRT) was administered to 133 patients. EFI field was used for treatment of 26 patients who received radiotherapy alone and 62 who received CCRT.
ResultsThe median follow-up period was 60 months. The 2- and 5-year overall survival (OS) rates were 87.8% and 73.5%, respectively, and the 2- and 5-year disease-free survival rates were 81.7% and 75.0%, respectively, however, no survival differences were observed between the two treatment field groups. EFI tended to increase OS in the radiotherapy alone group, but not in the CCRT group.
IntroductionThe spread of cervical cancer usually exhibits stepwise progression, from regional pelvic lymph nodes to para-aortic lymph nodes (PAN), followed by distant metastases with the prevalence of PAN metastases increasing progressively with stage [1,2]. Extended field irradiation (EFI) has therefore been utilized for treatment of occult PAN metastases in patients with advanced cervical cancer [2-7]. One randomized trial found that EFI had no effect on locoregional tumor control, but showed an association with increased overall survival (OS) rate [3], whereas a second randomized trial found that EFI did not affect locoregional control or survival, but reduced the rates of PAN and distant metastases without pelvic failure [4]. In both studies, the decision to treat PAN was difficult, because of significant late complications.
The use of concurrent chemoradiotherapy (CCRT) has complicated findings regarding the efficacy and safety of EFI [7-10]. In a comparison between EFI without chemotherapy and pelvis only field with CCRT in patients with locally advanced cervical cancer, CCRT significantly improved survival rate but had no effect on late toxicity [8]. In contrast, many retrospective studies have shown that EFI with CCRT is effective: thus, whether the combination of EFI with CCRT is effective or not because of associated toxicity is unclear [2,9,11-13].
This study therefore assessed the efficacy of EFI in patients with locally advanced cervical cancer without PAN involvement.
Materials and Methods1. Patient characteristicsBetween January 1996 and December 2010, 241 patients with locally advanced cervical cancer underwent radiotherapy as primary treatment at Keimyung University Dongsan Medical Center. Of 241 patients, 14 patients with an incomplete course of radiotherapy, 20 patients without regular follow-up after completion of radiotherapy, and four patients with neuroendocrine carcinoma were excluded, and 203 patients were analyzed in this study. Locally advanced cervical cancer was defined according to International Federation of Gynecology and Obstetrics (FIGO) staging as stage IB2, IIA (tumor size > 40 mm or pelvic lymph node metastases), IIB, IIIA, and IIIB [14]. Patient evaluation included medical history, pelvic examination, complete blood count, including hemoglobin concentration, liver and renal function tests, urinalysis, and chest radiography. Patients also underwent pelvic magnetic resonance imaging (MRI) or computed tomography (CT) for evaluation of tumor size and lymph node status. Malignancy criteria for lymph node metastases were a lymph node with diameter of 1 cm or more, spherical shape or having central necrosis [15]. Radiologic examination showed that none of these patients had PAN metastases. None of the patients had undergone surgical evaluation for lymph node metastases. Performance status was evaluated according to the Eastern Cooperative Oncology Group (ECOG) score [16].
2. TreatmentsAll patients received external beam radiotherapy (EBRT), followed by high dose-rate brachytherapy. EBRT was delivered with 6 to 20 mega-voltage photon beams using 4-field box techniques. Of the 203 patients, 115 (56.7%) were treated with a pelvis only field, as irradiation of the entire pelvis with the L4-L5 interspace as the superior border. The L5-S1 interspace was considered the superior border in patients who were relatively older or in poor general condition. The L3-L4 interspace was considered the superior border in patients who had extensive pelvic lymph node involvement. The remaining 88 patients (43.3%) were treated with EFI, defined as irradiation of the entire pelvis and PAN area with continuous fields using 4-field box techniques. The superior border was extended to encompass sufficient PAN spaces; 42 patients with L2-L3 interspace as the superior border, 17 patients with L1-L2 interspace and 29 patients with T12-L1 interspace. Midline shield and field size reduction were adapted after 36 to 45 Gy of EBRT. The median total EBRT dose to the pelvis was 54 Gy, ranging from 43.2 to 54 Gy. The median dose to PAN in patients treated with EFI was 45 Gy, ranging from 36 to 45 Gy. Nineteen patients received 36 Gy, one patient received 41.4 Gy, and 68 patients received 45 Gy. Dose to the PAN area was decided according to patients' condition or disease status. Following EBRT, patients were treated with high dose-rate brachytherapy using 60Co or 192Ir sources; 60Co was used until October 1998 and 192Ir was used thereafter. Under local anesthesia, tandem and ovoids were inserted and the radioisotope was applied using a remote after loading system. Patients were treated twice weekly with 5 Gy per fraction at A-point. Median brachytherapy dose to A-point was 30 Gy, ranging from 20 to 35 Gy. Combining the EBRT dose with brachytherapy dose, median total biologically equivalent dose for a 2 Gy fraction to A-point was 85.8 Gy, ranging from 77 to 90.6 Gy. The median overall treatment time was 64 days, ranging from 52 to 90 days.
Platinum based chemotherapy regimens were administered concurrently to 133 patients (65.5%). Until 2002, 37 patients received two cycles of continuous infusions of 5-fluorouracil and cisplatin every four weeks. Forty-nine patients received three cycles of paclitaxel and carboplatin or cisplatin every three weeks until 2006 and, since then, 47 patients received cisplatin every week. None of the patients received consolidation chemotherapy after completion of CCRT, but 12 patients underwent radical hysterectomy.
During treatment, each patient's performance status and complete blood count were evaluated weekly. Red blood cell transfusions were administered to patients with hemoglobin levels below 10 g/dL. When the absolute neutrophil count was below 1,000/mm3 or the platelet count was below 50,000/mm3, treatment was delayed until the blood count recovered.
In summary, four different treatment modalities were used in this study: EFI with CCRT in 62 patients (30.5%), pelvis only field with CCRT in 71 patients (35.0%), EFI without chemotherapy in 26 patients (12.8%), and pelvis only field without chemotherapy in 44 patients (21.7%).
3. Follow-up and response evaluationAfter completion of treatment, patients were evaluated regularly by radiation oncologists and gynecologic oncologists. Responses were defined as follows: complete response (CR), disappearance of the gross tumor on pelvic examination or pelvic MRI or CT; partial response (PR), ≥ 30% reduction of the initial tumor volume; progressive disease (PD), a ≥ 20% increase in tumor volume or occurrence of a new lesion; and stable disease, neither sufficient shrinkage for PR nor sufficient increase for PD [17]. Failure patterns were classified according to two different categories: locoregional failure, any recurrences in the pelvis, including the primary cervix and pelvic lymph nodes; distant metastases, both PAN metastases and metastases to distant lymph nodes or organs. Treatment-related toxicities were graded according to Common Terminology Criteria for Adverse Events ver. 4.0.
4. Statistical analysisOS was calculated from the start of treatment to death from any cause or last follow-up visit. Disease-specific survival (DSS) was calculated from the start of treatment to death from disease or last follow-up visit. Disease-free survival (DFS) was calculated from the end of treatment to the date of disease failure or last follow-up visit. The Kaplan-Meier method was used for calculation of OS and DFS, and the log-rank test was used for evaluation of prognostic factors. The chi-square test was used for comparison of patient characteristics and other factors between two groups. A p < 0.05 was considered statistically significant. All statistical analyses were performed using IBM ver. 20.0.0 (IBM Co., Armonk, NY).
Results1. Patient characteristics
Table 1 shows the characteristics of the 203 included patients. The median age was 59 years and ranged from 28 to 83 years. Most patients had an ECOG performance status of 0 to 1. Pathologic examination showed that 179 patients (88.2%) had squamous cell carcinomas, 16 patients (7.9%) had adenocarcinomas, six patients (3.0%) had adenosqua-mous carcinomas, and two patients (1.0%) had poorly differentiated carcinomas. Patients in the EFI and pelvis only field groups differed with regard to age, pelvic lymph node involvement, and tumor size. In the EFI group, patients were younger and a higher percentage of patients had pelvic lymph node involvement and large (> 40 mm) tumor size.
2. Response and patterns of failureThree months after completion of treatment, 197 patients (97.0%) achieved CR and six (3.0%) achieved PR, with similar response rates in patients treated with EFI and pelvis only field (p=0.615). The median follow-up period was 60 months (range, 4 to 184 months). At the time of the last follow-up, 133 patients (65.5%) were alive without evidence of disease, three (1.5%) were alive with disease, 46 (22.7%) had died from cervical cancer, and 21 (10.3%) had died from other causes.
Disease failure was observed in 51 patients (25.1%). Table 2 shows patterns of failure. Locoregional failures were observed in 27 patients (13.3%), including 15 with local failure including the cervix or vagina, and two with regional failure, defined as recurrences within the pelvic irradiation fields. Four patients showed both local and regional failure. Six patients who achieved PR after completion of treatment were included in locoregional failure. Twenty-nine patients (14.3%) had distant metastases, including three with PAN metastases, 22 with metastases to supraclavicular nodes or distant organs, and four with both. Of these 29 patients, 12 had been treated with EFI and 17 with pelvis only field. No statistically significant differences were observed between the two groups (p=0.590). Para-aortic metastases were observed in seven patients (3.4%), four treated with EFI and three with pelvis only field, however, the occurrence rates did not differ significantly between the two treatment groups (p=0.454). Of the patients who experienced distant metastases, nine had pulmonary metastases; four had hepatic metastases; four had distant lymph node metastases, including metastases to the left and right supraclavicular nodes; three had bone metastases; three had carcinomatosis peritonei; two had brain metastases; and one had transverse colon metastases. Disease failure was observed in 20 patients (22.7%) in the EFI group and in 31 (27.0%) in the pelvis only field group (p=0.495). Regarding patterns of failure in the two groups, we observed no significant between-group differences in locoregional, PAN, and distant failures.
3. SurvivalThe 2- and 5-year OS rates were 87.8% and 73.5%, respectively. The 2- and 5-year DSS rates were 89.6% and 78.7%, respectively. The 2- and 5-year DFS rates were 81.7% and 75.0%, respectively. Patients treated with EFI had 2- and 5-year OS rates of 87.0% and 71.7%, whereas patients treated with pelvis only field had 2- and 5-year OS rates of 88.4% and 74.8%, respectively (p=0.699) (Fig. 1). Similar 2- and 5-year DSS rates were observed for patients treated with EFI (90.3% and 77.4%, respectively) and pelvis only field (89.2% and 79.5%, respectively) (p=0.791). Similar 2- and 5-year DFS rates were also observed for patients treated with EFI (82.2% and 75.8%, respectively) and pelvis only field (81.4% and 74.5%, respectively) (p=0.668).
When analyzed patients treated with radiotherapy alone without concurrent chemotherapy, the 2- and 5-year OS rates were 92.1% and 72.1% in patients treated with EFI, and 75.0% and 60.5%, respectively, in patients treated with pelvis only field (p=0.056) (Fig. 2). The 2- and 5-year DSS rates were 95.8% and 82.8% in patients treated with EFI and 76.7% and 69.0%, respectively, in patients treated with pelvis only field (p=0.078). The 2- and 5-year DFS rates were 87.5% and 74.6% in patients treated with EFI and 72.4% and 63.6%, respectively, in patients treated with pelvis only field (p=0.110).
In patients treated with CCRT, however, treatment field had no significant effect on OS or DFS. The 2- and 5-year OS rates were 84.9% and 72.3% in patients treated with EFI, and 97.1% and 84.3%, respectively, in patients treated with pelvis only field (p=0.140) (Fig. 3). The 2- and 5-year DSS rates were 88.0% and 75.0% in patients treated with EFI and 97.1% and 86.2%, respectively, in patients treated with pelvis only field (p=0.175). The 2- and 5-year DFS rates were 80.0% and 78.0% in patients treated with EFI and 87.0% and 81.3%, respectively, in patients treated with pelvis only field (p=0.379).
Fig. 4 shows OS curves for the four treatment modalities. Significantly lower OS was observed in patients treated with pelvis only field without chemotherapy than in the other three groups (p=0.001). However, no significant intergroup differences in OS were noted among the other three treatment modalities.
4. Prognostic factors
Table 3 shows the results of univariate analysis for prognostic factors associated with OS and DFS. Good ECOG performance status (p=0.003), low FIGO stage (p=0.043), and use of concurrent chemotherapy (p=0.001) were prognostic factors for prolonged OS. All three factors remained statistically significant in multivariate analyses. Squamous cell pathology (p=0.018) and use of concurrent chemotherapy (p=0.035) were the only prognostic factors for DFS.
5. ToxicitiesA summary of acute and late grade 3-4 toxicities is shown in Table 4. Acute grade 3-4 toxicities were observed in 11 patients (5.4%). Nine patients (4.4%) experienced hematologic toxicities, including anemia and neutropenia, but all patients were properly managed and recovered sufficiently to continue treatment. Two patients (1.0%) experienced grade 3 diarrhea and received treatment as inpatients. No serious acute genitourinary toxicities were noted. Late grade 3-4 toxicities were observed in six patients (3.0%). Two patients (1.0%) experienced late gastrointestinal toxicities: one was treated with pelvis only field and experienced perforation of the small intestine and one was treated with EFI and experienced rectal perforation. Both patients were managed surgically. Four patients (2.0%) experienced late genitourinary toxicities. One patient experienced urinary tract obstruction and still remains in stent insertion state, whereas the other three experienced grade 3 hematuria.
No significant differences in the rates of acute or late grade 3-4 toxicities were observed between patients treated with EFI and pelvis only field. Late toxicities occurred in the entire pelvic field, not in the PAN area. Treatment with CCRT had no significant effect on toxicity rates.
DiscussionIn recent decades, radiotherapy has become the standard treatment for patients with locally advanced cervical cancer. EBRT followed by intracavitary brachytherapy was the standard treatment until the 1990s; since then, CCRT has become the standard treatment method [7,18-22]. To date, however, the role of EFI has not been clearly established, especially in patients undergoing CCRT [3,7]. In the absence of concurrent chemotherapy, EFI significantly enhanced OS [3]. The 5- and 10-year OS rates were estimated to be 67% and 55% for patients treated with EFI, and 55% and 44%, respectively, for patients treated with pelvis only field. Although a second randomized study found no significant difference in OS, the rate of distant metastases with local control was 2.4-fold greater and the rate of PAN metastases was 2.8-fold greater in patients treated with pelvis only field than in patients treated with EFI [4].
The results observed in the absence of concurrent chemotherapy were similar to these earlier findings. Higher 5-year OS rates were observed in patients treated with EFI than in patients treated with pelvic irradiation alone (72.1% vs. 60.5%). In the absence of concurrent chemotherapy, although the difference was marginally significant (p=0.056), prophylactic irradiation of the PAN area may be effective if toxicity is not a concern. However, in a previous randomized trial, the cumulative incidence of grade 4 and 5 toxicities was higher in patients treated with EFI (8% vs. 4%), with the difference being much greater in patients who had surgical staging or had undergone previous abdominal surgery [3]. Similarly, a second randomized trial showed that severe digestive complications were 2.3-fold more frequent in patients treated with EFI [4]. In this study, however, the incidence of toxicities did not differ significantly.
A trial comparing EFI without chemotherapy and pelvis only field with CCRT using 5-fluorouracil and cisplatin in patients with locally advanced cervical cancer found that OS and DFS were longer in the latter group, with 5-year OS rates of 52% and 73%, respectively [8]. However, the rates of para-aortic failure did not differ between these two groups, and OS benefits were statistically significant only in stage IB to IIB patients. Several studies have evaluated the efficacy of EFI with CCRT [6,10-13,23]. A phase I/II trial found that concurrent extended field CCRT with high dose-rate brachytherapy yielded a 5-year OS rate of 77%, with serious bowel toxicity observed in 6% of patients, suggesting that this regimen is safe and effective [12]. In this study, concurrent chemotherapy significantly increased OS compared with radiotherapy alone (p=0.001), however, treatment field had no benefit, with 5-year OS rates of 72.3% in patients treated with EFI and 84.3% in patients treated with pelvis only field (p=0.140).
The toxicity findings in patients treated with EFI plus CCRT are conflicting [2]. One trial reported acute hematologic toxicity and acute gastrointestinal toxicity rates of 10% and 2%, whereas another reported rates of 77.5% and 25%, respectively [3,20]. In this study, 4.8% of patients who received EFI with CCRT had acute hematologic and 1.6% had acute gastrointestinal toxicities, with no difference in toxicity rates according to treatment field. Only one patient experienced late toxicity, compared with 2% to 14% of patients in previous studies [2].
Patients in this study were treated with four different treatment modalities: EFI with or without concurrent chemotherapy, pelvis only field with or without concurrent chemotherapy. Significantly lower OS was observed in patients treated with pelvis only field without chemotherapy than in the other three groups. These results suggest that EFI may be reliable in the absence of chemotherapy by reducing the risk of systemic metastases. When combined with CCRT, however, the efficacy of EFI seems to be decreased, because chemotherapy has cytotoxic effects in controlling micrometastases of PAN and has radiation sensitizing effects in controlling pelvic disease [24].
This study was designed to show the efficacy of EFI and the results shed much light on the use of EFI. However, there were still some limitations, including the retrospective design. Because this study was not prospective, patients were not well matched in the treatment field groups and CCRT groups. Patients treated with EFI tended to be younger, positive for pelvic node metastases, and to have large-sized tumors. Patients treated with CCRT also tended to be younger and positive for pelvic node metastases. In addition, each group included a different number of patients, making comparisons difficult. In addition, chemotherapeutic regimens differed. From January 2001 to December 2002, 37 patients were treated with 5-fluorouracil and cisplatin. From January 2003 to December 2006, 49 patients were treated with paclitaxel and carboplatin or cisplatin after several studies demonstrated the advantages of these regimens [25]. Since January 2007, 47 patients have received weekly cisplatin on the basis of clinical findings [19,20]. Although no significant differences in survival were observed among patients receiving these chemotherapeutic regimens, the differences in these regimens may have affected our results. In addition, our evaluation of toxicities was limited. Because this study was retrospective in design and medical records were incomplete, we may have underestimated toxicities.
ConclusionWe found that EFI did not have a significant impact on survival outcomes in locally advanced cervical cancer patients without PAN involvement. Although this study was conducted retrospectively and had some limitations, these results may be useful when determining the optimal radiation treatment fields in patients. In the absence of CCRT, EFI may be appropriate; however, in patients administered concurrent chemotherapy, EFI may not be effective. Conduct of well-designed prospective or case matched studies will be necessary in order to confirm these results.
Fig. 2.Overall survival curves according to treatment field in patients treated with radiotherapy alone. EFI, extended field irradiation. 
Fig. 3.Overall survival curves according to treatment field in patients treated with radiotherapy alone. EFI, extended field irradiation. 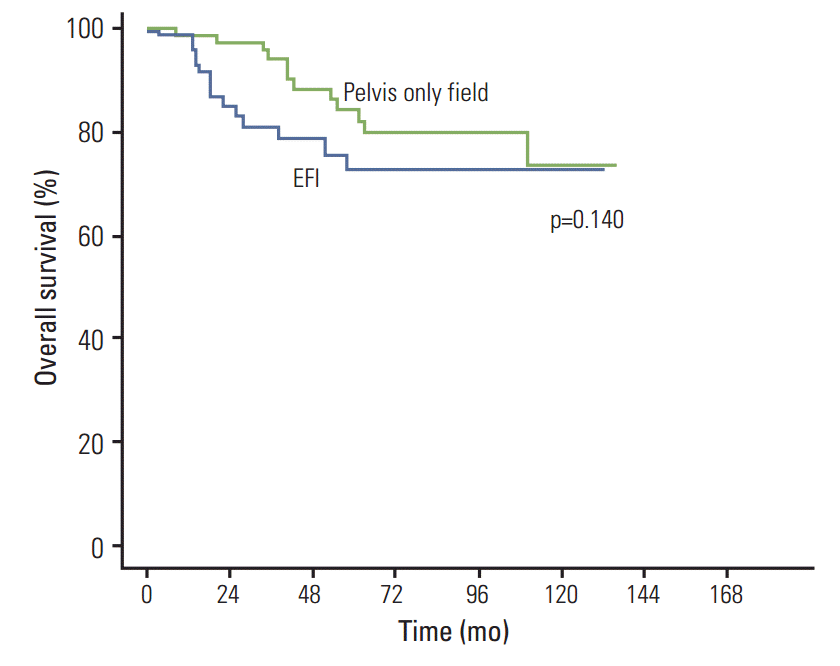
Fig. 4.Overall survival curves according to four different treatment modalities. EFI, extended field irradiation; CCRT, concurrent chemoradiotherapy. 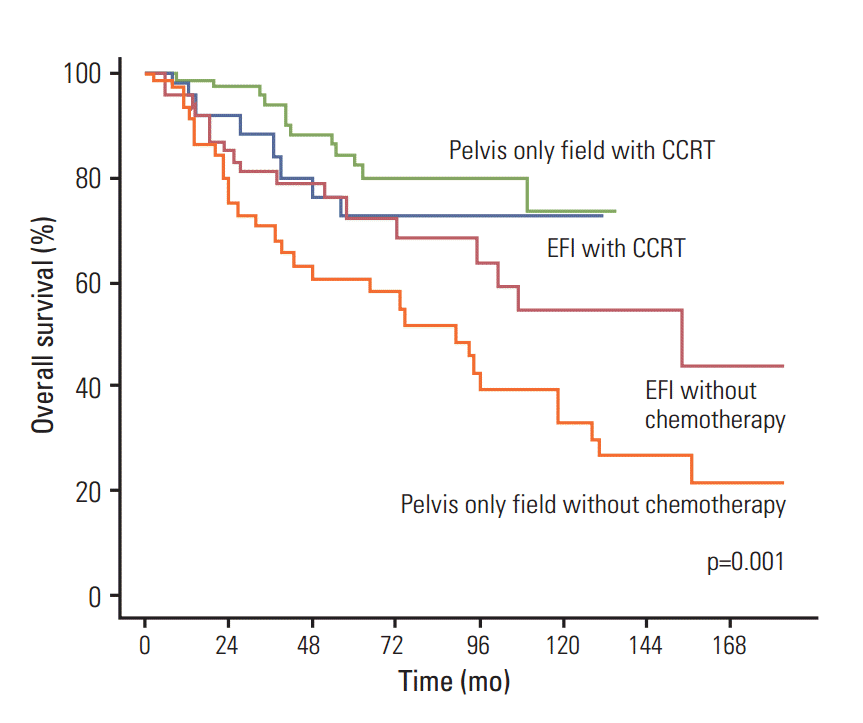
Table 1.Baseline demographic and clinical characteristics Table 2.Patterns of failure
Table 3.Univariate analyses of prognostic factors for overall survival (OS) and disease-free survival (DFS) References1. Berman ML, Keys H, Creasman W, DiSaia P, Bundy B, Blessing J. Survival and patterns of recurrence in cervical cancer metastatic to periaortic lymph nodes (a Gynecologic Oncology Group study). Gynecol Oncol. 1984;19:8–16.
2. Ring KL, Young JL, Dunlap NE, Andersen WA, Schneider BF. Extended-field radiation therapy with whole pelvis radiotherapy and cisplatin chemosensitization in the treatment of IB2-IIIB cervical carcinoma: a retrospective review. Am J Obstet Gynecol. 2009;201:109.e1-6.
3. Rotman M, Pajak TF, Choi K, Clery M, Marcial V, Grigsby PW, et al. Prophylactic extended-field irradiation of para-aortic lymph nodes in stages IIB and bulky IB and IIA cervical carcinomas. Ten-year treatment results of RTOG 79-20. JAMA. 1995;274:387–93.
4. Haie C, Pejovic MH, Gerbaulet A, Horiot JC, Pourquier H, Delouche J, et al. Is prophylactic para-aortic irradiation worthwhile in the treatment of advanced cervical carcinoma? Results of a controlled clinical trial of the EORTC radiotherapy group. Radiother Oncol. 1988;11:101–12.
5. Chatani M, Matayoshi Y, Masaki N, Narumi Y, Teshima T, Inoue T. Prophylactic irradiation of para-aortic lymph nodes in carcinoma of the uterine cervix: a prospective randomized study. Strahlenther Onkol. 1995;171:655–60.
6. Sood BM, Gorla GR, Garg M, Anderson PS, Fields AL, Runowicz CD, et al. Extended-field radiotherapy and high-dose-rate brachytherapy in carcinoma of the uterine cervix: clinical experience with and without concomitant chemotherapy. Cancer. 2003;97:1781–8.
7. Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–43.
8. Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol. 2004;22:872–80.
9. Thomas G, Dembo A, Fyles A, Gadalla T, Beale F, Bean H, et al. Concurrent chemoradiation in advanced cervical cancer. Gynecol Oncol. 1990;38:446–51.
10. Grigsby PW, Graham MV, Perez CA, Galakatos AE, Camel HM, Kao MS. Prospective phase I/II studies of definitive irradiation and chemotherapy for advanced gynecologic malignancies. Am J Clin Oncol. 1996;19:1–6.
11. Malfetano JH, Keys H, Cunningham MJ, Gibbons S, Ambros R. Extended field radiation and cisplatin for stage IIB and IIIB cervical carcinoma. Gynecol Oncol. 1997;67:203–7.
12. Chung YL, Jian JJ, Cheng SH, Hsieh CI, Tan TD, Chang HJ, et al. Extended-field radiotherapy and high-dose-rate brachytherapy with concurrent and adjuvant cisplatin-based chemotherapy for locally advanced cervical cancer: a phase I/II study. Gynecol Oncol. 2005;97:126–35.
13. Uno T, Mitsuhashi A, Isobe K, Yamamoto S, Kawakami H, Ueno N, et al. Concurrent daily cisplatin and extended-field radiation therapy for carcinoma of the cervix. Int J Gynecol Cancer. 2008;18:80–4.
14. Pecorelli S, Zigliani L, Odicino F. Revised FIGO staging for carcinoma of the cervix. Int J Gynaecol Obstet. 2009;105:107–8.
15. Akin O, Mironov S, Pandit-Taskar N, Hann LE. Imaging of uterine cancer. Radiol Clin North Am. 2007;45:167–82.
16. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
17. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
18. Rose PG, Bundy BN, Watkins EB, Thigpen JT, Deppe G, Maiman MA, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–53.
19. Keys HM, Bundy BN, Stehman FB, Muderspach LI, Chafe WE, Suggs CL 3rd, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–61.
20. Whitney CW, Sause W, Bundy BN, Malfetano JH, Hannigan EV, Fowler WC Jr, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to radiation therapy in stage IIB-IVA carcinoma of the cervix with negative para-aortic lymph nodes: a Gynecologic Oncology Group and Southwest Oncology Group study. J Clin Oncol. 1999;17:1339–48.
21. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–13.
22. Green JA, Kirwan JM, Tierney JF, Symonds P, Fresco L, Collingwood M, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet. 2001;358:781–6.
23. Varia MA, Bundy BN, Deppe G, Mannel R, Averette HE, Rose PG, et al. Cervical carcinoma metastatic to para-aortic nodes: extended field radiation therapy with concomitant 5-fluorouracil and cisplatin chemotherapy: a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1998;42:1015–23.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||