Introduction
Metastasis of breast cancer is usually diagnosed on clinical, biological, and radiologic findings. Although histopathological proof of metastasis is not usually required, a suspicious metastatic lesion is occasionally revealed to be benign, confirmed as an inflammation or infection, instead of carcinoma. Additionally, a particular concern is being made to confirm that the lesion is a metastasis of the primary known cancer, and to reassess tumor’s characteristics due to a discrepancy in the hormonal receptor and HER2 status between primary and metastatic breast cancer. The different biologic status between the primary tumor and metastases led to altered management in 20% of patients [1]. Accordingly, a biopsy of suspicious metastatic lesion is recommended. Among various benign lesions, which can be regarded as metastasis, there have been several case reports of sarcoidosis in many different cancer patients. The link between sarcoidosis and carcinogenesis is unclear; however, immune dysfunction may facilitate both disorders [2]. In this article, we describe a case of sarcoidosis in a patient with breast cancer that could have been mistaken for metastatic disease.
Case Report
A 44-year-old woman with a history of breast cancer was presented to out institution with abnormal findings on 18-fluorodeoxyglucose (FDG)-positron emission tomography(PET) imaging that was performed at another institution. She had previously been diagnosed with breast cancer (T1N2M0, Stage IIIa, intraductal carcinoma, triple negative cancer).
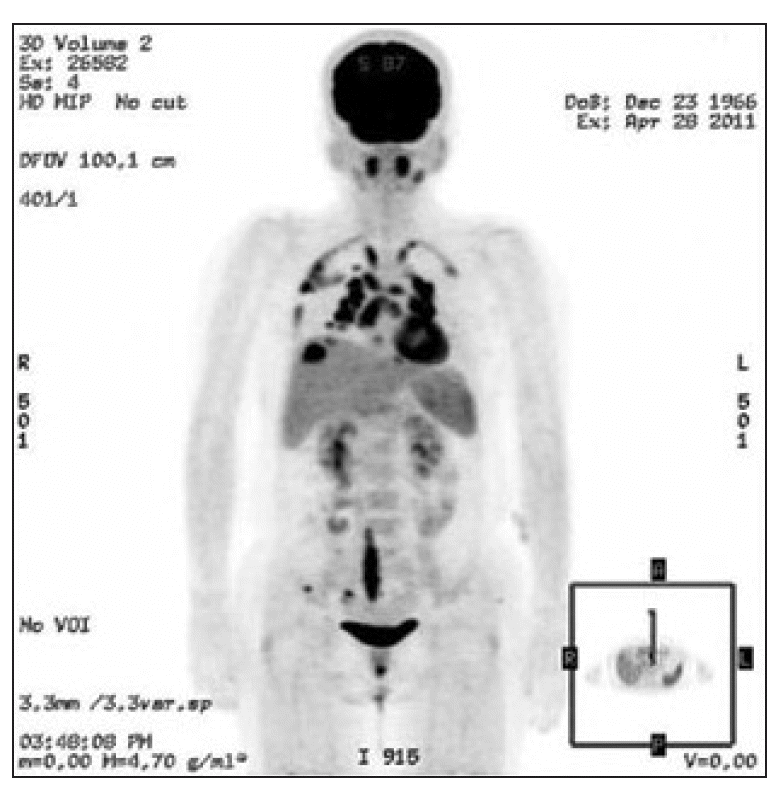
She had undergone adjuvant chemotherapy and radiation therapy after a right breast conserving surgery 2 years ago. She was a non-smoker and had no specific occupational or environmental exposure to known carcinogens. She had been taking tamoxifen after chemotherapy. Her FDG-PET scan demonstrated multiple new hypermetabolic lesions on the parenchyma of both lungs (standardized uptake value [SUV], 11.5) and both thoracic pleura (SUV, 9.0). FDG-PETcomputed tomography (CT) scan also demonstrated a new intense FDG uptake in both the paratracheal lymph nodes (SUV, Rt. 8.4/Lt 7.1), lymph nodes in the AP window (SUV, 9.6), subcarinal lymph nodes (SUV, 8.6), both hilar lymph nodes (SUV, Rt. 10.2/Lt. 13.7), both interlobar lymph nodes (SUV, Rt. 10.7/Lt. 13.2), and paraesophageal lymph nodes (SUV, 4.8) (Fig. 1).
Laboratory results were all within normal limits, except for mild anemia (hemoglobin 11.4 mg/dL) and leucopenia (3,300/μL). Chest CT scans revealed faint ground-glass opacities (GGO) and fine reticular densities on both the upper subpleural lung and multifocal areas of the lower lungs (Fig. 2). Photograph (Fig. 2A) in coronal plane and (Fig. 2C) in axial plane of CT scan, photograph (Fig. 2C) shows GGO on lung setting. The scan also demonstrated small nodules in the right lung, as well as multiple lymph node enlargements in both the hilum and mediastinum that were symmetrically distributed.
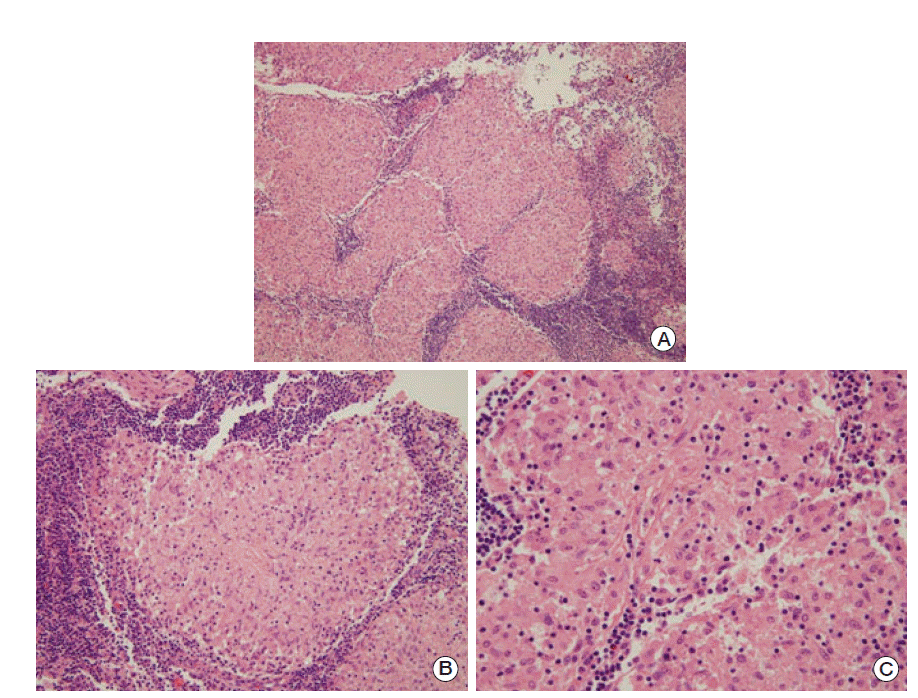
A formal multistep evaluation was performed to evaluate the GGO of the lung parenchyma and the multiple mediastinal lymphadenopathies. FDG-PET/CT scans are one of the most useful screening tools for possible distant metastases. According to the chest CT scan, we concluded that metastasis would be unlikely as there existed GGO around the mediastinal lymph node. However, we were not able to completely rule out metastasis; therefore, we conducted a mediastinoscopic biopsy. Histological analysis of the mediastinal lymph node biopsy demonstrated a chronic, non-caseating, granulomatous inflammation (Fig. 3; photomicrograph shows well-formed noncaseating granulomas with Langerhans and foreign body-type giant cells, hematoxylin phloxine saffron stain; original magnification ×100).
Additional laboratory findings supported the diagnosis of sarcoidosis, rather than tumor or tuberculosis. Her purified protein derivative skin test for tuberculosis was negative, and the biopsy sample was negative for tuberculosis antigenspecific interferon gamma. Her angiotensin I-converting enzyme level was elevated to 101 U/L. Additional imaging studies, including an abdominal CT scan and bone scan, did not show any evidence of metastatic disease or involvement of sarcoidosis. Because the patient did not complain of any respiratory symptom, her sarcoidosis was left untreated. She could avoid unnecessary chemotherapy, unless she had avoided operation.
Discussion
Many patients with metastatic breast cancer are presented with nonspecific symptoms, such as new pain, weight loss, or dyspnea. Whenever possible, tissue acquisition for diagnostic confirmation and reassessment of receptor status (estrogen receptor, progesterone receptor, and HER2) should be considered. However, in an actual clinical setting, certain imaging findings, such as multiple areas of osseous lytic or blastic metastases or multiple sites with visceral involvement, may be considered as acceptable evidence for metastatic disease without the requirement for tissue confirmation.
FDG-PET scan is one of the optional imaging modalities that can be used to evaluate metastatic disease in cancer patients. Unfortunately, however, limited studies support a potential role for FDG-PET/CT to detect regional node involvement, as well as distant metastasis in locally advanced breast cancer [3]. With regard to high metabolic activity on FDG-PET scans in many nonmalignant conditions with active granuloma formation, such as sarcoidosis, tuberculosis, nontuberculous mycobacterium, and fungal infections, the consensus of the National Comprehensive Cancer Network (NCCN) Panel is that FDG-PET/CT is optional, which is applied to situations where standard staging studies are equivocal or suspicious, especially in the setting of locally advanced or metastatic disease [4]. In one retrospective study, the features of hilar foci of the benign pulmonary nodules (group III) were similar between patients with cancer and those with no metastatic disease. The SUV max of the malignant lesions was significantly higher (6.6±4.1) than that of the lesions with a benign etiology (3.5±1.0) (p < 0.001). However, the SUV max of granulomatous disease was as high as that of malignant disease [5].
In our case, the patient had a moderately high possibility of metastatic disease given her stage of breast cancer; women with triple negative breast cancer experience the peak risk of recurrence within 3 years of diagnosis, achieving around 20 percent of disease recurrence at 5 years [6]. In practice, following the aforementioned NCCN guideline, it is not recommended to take a FDG-PET scan in situations whereby the results of the chest CT scan were not typical of metastatic disease. Because the preexisting result from FDG-PET scan was suggestive of metastasis, we conducted a mediastinoscopic lymph node biopsy to confirm.
Occasionally, the granulomatous tissue in a sarcoid-like reaction is found to be surrounding many types of solid organ tumors. Four hundred seventy-four women were followed-up to determine the prevalence of breast cancer and sarcoidosis. Breast cancer was observed in only 2 cases of these patients, whereas 7.6 were expected in the general population; however, there was no relationship between the presence of sarcoidosis and the incidence of breast cancer, indicating that sarcoidosis may precede, accompany, or follow the diagnosis of breast cancer [7]. Sarcoid reactions in the lymph drainage or tumor tissue may be found in breast cancer, but with rarity. A literature search resulted in a total of nine case reports regarding breast cancer and sarcoidosis; however, for two of these studies, neither the abstract nor the article could be obtained [8,9]. The remaining seven case reports were of pulmonary sarcoidosis mimicking metastatic breast cancer [10-12].
Research groups in Japan and Europe reported an increased risk of malignancy in patients with sarcoidosis [13,14]. However, other researchers failed to demonstrate a higher risk for malignancy in patients with sarcoidosis [15]. As of yet, there has been no conclusive correlation between sarcoidosis and breast cancer. Additionally, the effects of cytotoxic chemotherapeutics or drugs for depressive disorder on the development of sarcoidosis are unknown. Furthermore, there are a few identified risk factors for sarcoidosis.
Although pulmonary sarcoidosis in breast cancer patients is a rare phenomenon, clinicians could encounter many similar benign conditions. Our case highlights the need to consider non-malignant diagnoses in those with prior malignancies, and the need to evaluate whenever possible the first recurrence of a tumor following potentially curative therapy. We recommend performing biopsy sampling to rule out metastatic disease in addition to imaging studies.