AbstractPurposeSystemic chemotherapy is the only option for patients with unresectable/metastatic hepatocellular carcinoma (HCC) who are not candidates for local/regional treatment. However, the response to such treatment and survival are poor, especially in hepatitis B virus (HBV) endemic areas. The aim of this study was to determine the efficacy of cisplatin-based combination chemotherapy and identify a subgroup of advanced HCC patients with favorable responses.
Materials and MethodsThe medical records of all consecutive patients with unresectable/metastatic HCC who received cisplatin-based combination chemotherapy between January 2003 and October 2009 were reviewed. Time to progression (TTP) and overall survival (OS) were determined using Kaplan-Meier analysis. Univariate and multivariate analyses were performed to identify prognostic factors for TTP and OS.
ResultsData for 46 patients were analyzed. First-line chemotherapies consisted of cisplatin-based combination treatment with doxorubicin, fluoropyrimidines and gemcitabine. The response rate for all patients was 4.3%. The median TTP and OS were 1.8 (95%confidence interval [CI], 1.1 to 2.5) and 7.2 (95% CI, 3.0 to 11.5) months, respectively. Eastern Cooperative Oncology Group (ECOG) performance status (PS), Child classification, Cancer of the Liver Italian Program (CLIP) score and portal vein thrombosis (PVT) were identified by univariate analyses as prognostic factors for TTP and OS. ECOG PS (hazard ratio [HR], 4.51; 95% CI, 1.61 to 12.6; p=0.004) and PVT (HR, 2.12; 95% CI, 1.10 to 4.11; p=0.026) were independent prognostic factors for TTP.
IntroductionPatients with unresectable or metastatic hepatocellular carcinoma (HCC), who are not candidates for local/regional treatment, have a very limited number of therapeutic options (1). To date, sorafenib is the only systemic therapy proven to prolong overall survival in patients with advanced HCC who are not candidates for surgical or local/regional therapies. However, the median survival achieved with sorafenib therapy was reported to be only 10.7 months compared with 7.9 months in the placebo arm (hazard ratio [HR], 0.69; 95% confidence interval [CI], 0.55 to 0.87; p<0.001), and was even shorter in Asian patients (6.2 vs. 4.1 months; HR, 0.67; 95% CI, 0.49 to 0.93; p=0.0155) (2,3).
For those patients who are not candidates for sorafenib treatment or for those who have had treatment failures after sorafenib, the only therapeutic option is systemic chemotherapy. However, there are no standard chemotherapy regimens that have been shown to improve overall survival. A large number of controlled and uncontrolled studies have been performed with most of the major classes of chemotherapy agents given as single or combination therapies. Single or combination regimens of other drugs, including doxorubicin, 5-fluorouracil (5-FU), capecitabine, cisplatin, gemcitabine, and mitoxantrone have elicited 0% to 27% response rates, 2 to 6 months time to progression (TTP) or progression-free survival, and 3 to 12 months of overall survival (OS) (4-9).
The most widely studied regimens in patients with advanced HCC are doxorubicin and cisplatin-based chemotherapies. Doxorubicin was associated with a single agent response rate of 0-15% (4,10). Doxorubicin as a single agent, when compared to the combination of cisplatin, interferon, doxorubicin, and 5-FU (PIAF) in a phase III randomized trial, caused a lower response rate (10.5% vs. 20.9%) but a similar OS rate (6.83 vs. 8.67 months, p=0.83). Doxorubicin single agent used to be considered a standard treatment for patients with advanced HCC before the sorafenib era (11). Cisplatin-based combination regimens were reported in some studies to result in higher objective response rates than regimens excluding cisplatin. Cisplatin administration had an objective response rate of 15% as single-agent chemotherapy, and a higher response rate when combined with doxorubicin, 5-FU and gemcitabine (5-9,11,12). However, there are insufficient data to recommend any one regimen as the standard of care.
Systemic chemotherapy loses efficacy due to the frequently observed development of multidrug tumor resistance, which is related to the high rate of expression of the multidrug resistance gene (MDR1), and p53 tumor suppressor gene mutations (13,14). Poor hepatic function in patients with advanced HCC results in higher toxicity and precludes systemic chemotherapy in many patients. Therefore, patient selection for those who would benefit from systemic chemotherapy is of vital importance before initiating systemic chemotherapy in patients with advanced HCC.
The aim of this study was to determine the efficacy of cisplatin-based combination chemotherapy and identify a subgroup of patients with advanced HCC who would be candidates for cisplatin-based combination chemotherapy. The records of all consecutive patients with advanced HCC who received cisplatin-based combination chemotherapy at a single center, before the sorafenib era, were retrospectively analyzed by univariate and multivariate prognostic factor analyses.
Materials and Methods1. PatientsBetween January 2003 and October 2009, consecutive patients with unresectable or metastatic HCC who received cisplatin-based combination as the first-line chemotherapy at Seoul National University Bundang Hospital were retrospectively enrolled. Patients had disease progression after a curative resection or other local treatment procedures, such as radiofrequency ablation (RFA), cryoablation or transarterial chemoembolization (TACE), or were not amenable to local/regional treatment. The diagnosis of HCC was confirmed by histopathology or by computed tomography (CT), magnetic resonance imaging (MRI), and angiographic findings in addition to elevated values of alpha-fetoprotein (AFP) using the guidelines proposed by the Korea Liver Cancer Study Group (15). Using these criteria, a patient was diagnosed with HCC if one or more risk factors (hepatitis B virus [HBV] or hepatitis C virus [HCV] infection, or cirrhosis) were present, and one of the following was also present: a serum AFP level>400 ng/mL and a positive result on at least one of the three typical liver imaging techniques (spiral CT, contrast enhanced dynamic MRI or hepatic angiography); or a serum AFP level<400 ng/mL and positive findings on at least two of the three imaging techniques. A positive finding for typical HCC with dynamic CT or MRI is indicative of arterial enhancement followed by venous washout in the delayed portal/venous phase. TNM stages were used for tumor staging and clinical stages were classified according to the Cancer of the Liver Italian Program (CLIP) staging system (16,17).
2. Cisplatin-based combination chemotherapyThe first-line chemotherapy regimens given to patients were cisplatin-based combination chemotherapy with doxorubicin (AP), 5-FU (FP), capecitabine (XP) and gemcitabine (GP). The AP regimen consisted of doxorubicin 60 mg/m2 delivered as an intravenous infusion over 30 minutes on day 1, followed by cisplatin 60 mg/m2 infused over 30 minutes on day 1. The FP regimen consisted of 5-FU 1,200 mg/m2 administered continuously on days 1 to 4, and cisplatin 60 mg/m2 infused over 30 minutes on day 1. The XP regimen consisted of capecitabine 2,000 mg/m2 orally administered on days 1 to 14, and cisplatin 60 mg/m2 infused over 30 minutes on day 1. The GP regimen consisted of gemcitabine 1,200 mg/m2 infused on days 1 and 8, and cisplatin 60 mg/m2 infused over 30 minutes on day 1. Chemotherapy cycles were repeated every 21 days until disease progression or intolerable toxicity.
3. Response and toxicity evaluationResponse was assessed after every two cycles of chemotherapy by CT or MRI scan using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria (18). Complete response (CR) was defined as the disappearance of all target and non-target lesions compared to baseline. Partial response (PR) was defined as at least a 30% decrease in the sum of the longest diameters of all target lesions, taking as a reference the baseline sum of the diameters with no new lesions appearing. Patients were considered to have progressive disease (PD) if any new lesion appeared, if the tumor size increased by at least 20% in the sum of the diameters of the target lesions, taking as reference the smallest sum on study, or if there was unequivocal progression of existing non-target lesions. A patient who failed to meet the definition of CR, PR or PD was classified as having stable disease (SD). The percentage of patients who had the best responses (other than PD) according to the RECIST criteria, and had those responses maintained for at least 28 days after the first radiologic evaluation, was defined as the disease control rate. Toxic effects of chemotherapy were evaluated according to the National Cancer Institute-Common Terminology Criteria for Adverse Events version 3.0.
4. Statistical analysisThe TTP was calculated from the first day of chemotherapy to the day when progressive disease was documented. OS (in days) was calculated from the first day of chemotherapy to death by any cause. All survival distributions were calculated using the Kaplan-Meier method and the log-rank test was used for univariate analysis. The Cox proportional hazard model was used to assess the prognostic factors by multivariate analysis. All analyses were done using SPSS ver. 15.0 (SPSS Inc., Chicago, IL) and a p≤0.05 was considered statistically significant. The study was approved by an independent review board at the Seoul National University Bundang Hospital (IRB approval number: B-1003-095-102).
Results1. Patient characteristicsBetween January 2003 and October 2009, 73 patients received systemic chemotherapy at Seoul National University Bundang Hospital. Among the 73, we identified 46 who underwent cisplatin combination chemotherapy as the first-line chemotherapy. The diagnosis of HCC was confirmed by pathology in five patients; for the remaining 41 patients, the diagnosis was established by CT, MRI, and angiographic findings in addition to elevated values of AFP using the guidelines proposed by the Korea Liver Cancer Study Group (15).
A total of 46 patients with HCC were analyzed in this study, and their clinical characteristics are summarized in Table 1. The mean age of the patients was 53 years (range, 21 to 73 years) and the male to female ratio was 4.1 : 1. HBV infection was documented in most of the patients (82.6%), HCV infection in a few (4.3%) and both infections in even fewer (2.2%). The majority of patients had an Eastern Cooperative Oncology Group (ECOG) performance (PS) score of 0-1 (84.8%) with liver function classified as Child-Pugh classification A (69.6%). The sites of extrahepatic metastases included lung in 32 (69.6%), bone in 14 (30.4%) and lymph nodes in 31 (67.4%) patients. Previous treatments included surgery in 13 patients (28.3%), TACE in 31 (67.4%), and RFA in six patients (13.0%). An AFP level higher than 400 ng/mL was recorded in 23 (50.0%) patients, and 28 patients (60.9%) had portal vein thrombosis. The HBeAg was positive in 10 patients (25.6% among HBV-positive patients). Eleven patients (23.9%) had a CLIP score of 0 or 1 points, 23 (50.0%) patients had 2-3 points, and 12 patients (26.1%) had 4-6 points.
2. EfficacyPatients received a mean of 2.3 cycles of cisplatin-based combination chemotherapy (range, 1 to 6 cycles): 10 patients received doxorubicin combination (AP); 32 fluoropyrimidine combination (FP and XP); and 4 patients received gemcitabine combination (GP) chemotherapy. The best responses to first-line chemotherapy are shown in Table 2: no complete response was observed; two patients (4.3%) achieved partial remission; 14 (30.4%) had stable disease; and 30 (65.2%) had progressive disease. The overall response rate was 4.3% and the disease control rate was 34.7%. There were no significant differences in response rates among the different regimens (p=0.28).
A total of 19 patients received second-line chemotherapy. A mean of 2.8 cycles of chemotherapy (range, 1 to 6 cycles) was given. Two patients received anthracycline-based chemotherapy, 4 fluoropyrimidine-based, 10 gemcitabine-based, and 3 patients received sorafenib. The response rate was 5.3% (1/19) and the disease control rate was 47.4% (9/19).
3. Survival analysisAfter a median follow-up duration of 5.5 months, the median time to progression for all patients was 1.8 months (95% CI, 1.1 to 2.5). There was no statistically significant difference in the time to progression among the regimens: 2.3 (95% CI, 0.2 to 4.4) months for the AP regimen, 1.8 months (95% CI, 0.8 to 2.2) for the fluoropyrimidine combination (FP, XP) and 1.9 (95% CI, 0.1 to 2.5) months for the GP regimen. The median OS from the start of chemotherapy was 7.2 (95% CI, 3.0 to 11.5) months. The overall survival was 8.5 (95% CI, 3.0 to 13.9) months for the AP regimen, 4.1 months (95% CI, 2.3 to 5.8) for the fluoropyrimidine combination and 4.7 months (95% CI, cannot be calculated) for the GP regimen (Table 2).
4. Prognostic factorsOn univariate analysis, poor ECOG PS, Child classification B compared to A, high CLIP score, and presence of portal vein thrombosis were statistically significant factors that indicated a poor prognosis for both TTP and OS (Table 3) (Fig. 1). Based on multivariate analyses using a model with factors entered for a significance level of p≤0.05, poor ECOG PS (HR, 4.51; 95% CI, 1.61 to 12.6; p=0.004) and the presence of portal vein thrombosis (HR, 2.12; 95% CI, 1.10 to 4.11; p=0.026) were independent poor prognosis factors for TTP. The presence of portal vein thrombosis (HR, 2.77; 95% CI, 1.36 to 5.62; p=0.005) was the only significant poor prognosis factor for OS (Table 4).
5. ToxicityToxicities were mostly hematologic, with grade 3/4 leukopenia in six (13%), neutropenia in 14 (30.4%) and thrombocytopenia in five (10.9%) patients. A Grade 3/4 elevation of the aminotransferases occurred in 11 (23.9%) patients, and jaundice in eight (17.4%) patients. There was no significant difference in hematologic and liver-related toxicities among the cisplatin-based combination (Table 5) treatment. However, grade 3/4 neutropenia was more frequent in the GP and AP regimens. There was no treatment-related death.
DiscussionThe results of this study confirmed the poor prognosis and poor response to cisplatin-based combination chemotherapy among patients with advanced HCC, who were not amenable to local/regional treatment outside of the clinical trial setting. In this study, cisplatin-based combination chemotherapy regimens had a response rate of 4.3%, a median TTP of 1.8 (95% CI, 1.1 to 2.5) months, and a median OS of 7.2 (95% CI, 3.0 to 11.5) months.
Our results are consistent with earlier studies that investigated the efficacy of cisplatin-based combination chemotherapy, although the results of our study are modest compared to prior studies with regard to TTP. The results of our study might more accurately reflect real clinical practice, with unselected patients. A high proportion of patients with metastases (89.1%) and portal vein thrombosis (60.9%) in this population may also explain the poor response rates. The combination of gemcitabine with cisplatin was reported to have a response rate of approximately 20% (7), a TTP of 18 weeks, and a median OS of 21 weeks. The combination of capecitabine and cisplatin elicited a response rate of 6.3%, a TTP of 2 months, and a median OS of 12.2 months (8).
Although not statistically significant, the AP regimen had a longer TTP and OS than the other regimens (FP, XP, GP) (Table 2). There were no differences in clinical characteristics of patients who received AP compared to the other regimens in this study. Lee et al. (6) reported that the response rate and disease control rate associated with the AP regimen were 18.9% (7/37) and 35.1% (13/37), respectively, and the median TTP and OS were 6.6 and 7.3 months. Kang et al. (12), found that the response rate and disease control rate of the AP regimen were, respectively, 58.6% (6/21) and 76.2% (46/21), and the median TTP and OS were, respectively, 5.4 and 10.7 months. The OS achieved with the AP regimen showed consistency across trials, 7.3-10.7 months, and compared favorably to the effects of sorafenib reported in an Asian trial (3). Further study comparing cisplatin-based combination chemotherapy, especially the AP regimen, with sorafenib or a combination of cytotoxic regimens with sorafenib is needed in patients with advanced HCC.
Based on the results of multivariate analysis, the only independent prognostic factors in this study population were ECOG PS (p=0.004) and portal vein thrombosis (p=0.026) with regard to TTP, and portal vein thrombosis (p=0.005) with regard to OS (Table 4). ECOG PS, Child-Pugh classification, CLIP score and portal vein thrombosis were prognostic factors for TTP and OS on univariate analysis. However, the Child-Pugh classification lost its statistical significance in the multivariate analysis, probably due to the small number of patients enrolled. The CLIP score was not included in the multivariate model, since the CLIP score represented a combination of other risk factors: Child-Pugh stage; tumor morphology and extension; serum AFP levels; and portal vein thrombosis.
The overall survival of patients associated with CLIP scores of 0-1, 2-3 and 4-6 were 13.3 (95% CI, 1.9 to 24.6), 7.6 (95% CI, 2.7 to 12.4) and 2.2 (95% CI, 1.6 to 2.7) months, respectively (Table 3). The median OS of patients in this study was shorter compared to OS reported in other studies (17,19-22). All patients had disease progression after a curative resection or other local treatment procedures by enrollment criteria, and the OS was assessed from day one of chemotherapy, not from the time of diagnosis, which may explain the short survival duration. However, the decrease in survival associated with high CLIP score remained consistent, validating the prognostic value of CLIP score in this advanced HCC population.
The results of this study are consistent with the findings of prior studies regarding prognostic factors (23-25). Leung et al. (23) reported that the prognosis for patients with unresectable HCC after systemic chemotherapy depended on pre-treatment liver function and the stage of disease. Portal vein thrombosis causes stenosis or occlusion of the portal vein; as a result, the blood supply to the liver parenchyma is decreased and further deterioration of liver function can occur. Zhang et al. (24) reported that portal vein thrombosis was often associated with a poor prognosis, and suggested palliative 3-dimensional conformal radiotherapy, targeting the main portal vein thrombosis. Tan et al. (25) analyzed clinical prognostic factors in 397 untreated patients with HCC and found that poor performance status, presence of ascites, and high AFP levels were statistically significant prognostic factors associated with decreased overall survival.
If the prognostic factors associated with TTP identified in our study were used to select candidates for cisplatin-based combination chemotherapy (ECOG PS 0-1 vs. 2, and presence of PVT), the patients who had no risk factors (ECOG PS 0-1 without PVT) would have shown a statistically significant increase in TTP compared to patients with 1 or 2 poor prognostic factors (3.1 [95% CI, 1.8 to 4.3] vs. 1.3 [95% CI, 1.3 to 1.4] vs. 0.7 [95% CI, 0.5 to 0.9] months, p<0.0001) and also in OS (14.5 [95% CI, 10.5 to 18.5] vs. 4.5 [95% CI, 0.0 to 9.8] vs. 2.2 (1.1 to 3.2) months, p<0.0001). These results suggest that patient selection based on the prognostic factors described above could aid in selecting candidates who would benefit from cisplatin-based combination chemotherapy. Fifteen patients (32.6%) in the current study had ECOG PS 0-1 and no PVT, and their median TTP and OS were, respectively, 3.1 months (95% CI, 1.8 to 4.3 months) and 14.5 months (95% CI, 10.5 to 18.5 months), which compares favorably with sorafenib data in an Asian trial (3). On the other hand, six patients (13%) with ECOG PS 2 and PVT in the current study had a very poor prognosis and could have been spared the toxicities of the cisplatin-based combination chemotherapy, if patient selection had been based on the above prognostic factors prior to commencing chemotherapy.
The limitations of our study include its retrospective nature. In addition, the selection of the chemotherapy regimen was not randomly assigned, but rather decided based on physician and patient preference, which could have led to biased results. Furthermore, the small sample size might have contributed to a lack of power in comparing the different chemotherapy regimens with regard to their toxicity, efficacy, and prognostic value.
Despite these limitations, our study confirmed the poor prognosis and efficacy of cisplatin-based combination chemotherapy in patients with advanced HCC in a real world setting. Our data suggest that selection of candidates for cisplatin-based combination chemotherapy based on prognostic factors - ECOG PS, and presence of PVT- would benefit select patients with advanced HCC, while sparing the others the unnecessary toxicities of combination chemotherapy. Careful classification of patients according to these prognostic variables should be part of the study design of future investigations of HCC chemotherapies.
ConclusionCisplatin-based combination chemotherapy in patients with advanced HCC showed a short TTP and a low response rate regardless of the chemotherapy regimen used. Systemic chemotherapy in patients with advanced HCC has limitations, however. Patients with a good ECOG performance status and absence of portal vein thrombosis had a longer TTP and OS, and therefore can be considered as good candidates for cisplatin-based combination chemotherapy.
References1. Nowak AK, Chow PK, Findlay M. Systemic therapy for advanced hepatocellular carcinoma: a review. Eur J Cancer. 2004;40:1474–1484. PMID: 15196530
2. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. PMID: 18650514
3. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. PMID: 19095497
4. Sciarrino E, Simonetti RG, Le Moli S, Pagliaro L. Adriamycin treatment for hepatocellular carcinoma: experience with 109 patients. Cancer. 1985;56:2751–2755. PMID: 2413981
5. Okada S, Okazaki N, Nose H, Shimada Y, Yoshimori M, Aoki K. A phase 2 study of cisplatin in patients with hepatocellular carcinoma. Oncology. 1993;50:22–26. PMID: 7678453
6. Lee J, Park JO, Kim WS, Park SH, Park KW, Choi MS, et al. Phase II study of doxorubicin and cisplatin in patients with metastatic hepatocellular carcinoma. Cancer Chemother Pharmacol. 2004;54:385–390. PMID: 15248028
7. Parikh PM, Fuloria J, Babu G, Doval DC, Awasthy BS, Pai VR, et al. A phase II study of gemcitabine and cisplatin in patients with advanced hepatocellular carcinoma. Trop Gastroenterol. 2005;26:115–118. PMID: 16512457
8. Lee JO, Lee KW, Oh DY, Kim JH, Im SA, Kim TY, et al. Combination chemotherapy with capecitabine and cisplatin for patients with metastatic hepatocellular carcinoma. Ann Oncol. 2009;20:1402–1407. PMID: 19502532
9. Ikeda M, Okusaka T, Ueno H, Takezako Y, Morizane C. A phase II trial of continuous infusion of 5-fluorouracil, mitoxantrone, and cisplatin for metastatic hepatocellular carcinoma. Cancer. 2005;103:756–762. PMID: 15637692
10. Lai CL, Wu PC, Chan GC, Lok AS, Lin HJ. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma: a prospective randomized trial. Cancer. 1988;62:479–483. PMID: 2839280
11. Yeo W, Mok TS, Zee B, Leung TW, Lai PB, Lau WY, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97:1532–1538. PMID: 16234567
12. Kang BL, Yuh YJ, Kim SR, Song HS, Lee SN, Shin DB, et al. A phase II study of doxorubicin and cisplatin combination chemotherapy for advanced hepatocellular carcinoma. Korean J Med. 2005;68:203–210.
13. Kong XB, Yang ZK, Liang LJ, Huang JF, Lin HL. Overexpression of P-glycoprotein in hepatocellular carcinoma and its clinical implication. World J Gastroenterol. 2000;6:134–135. PMID: 11819542
14. Katiyar S, Dash BC, Thakur V, Guptan RC, Sarin SK, Das BC. P53 tumor suppressor gene mutations in hepatocellular carcinoma patients in India. Cancer. 2000;88:1565–1573. PMID: 10738214
15. Park JW. Practice guideline for diagnosis and treatment of hepatocellular carcinoma. Korean J Hepatol. 2004;10:88–98. PMID: 15218342
16. American Joint Committee on CancerAJCC Cancer Staging Manual. 2002. 6th edNew York: Springer.
17. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. PMID: 9731568
18. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. European Organization for Research and Treatment of CancerNational Cancer Institute of the United StatesNational Cancer Institute of CanadaNew guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. PMID: 10655437
19. The Cancer of the Liver Italian Program (CLIP) InvestigatorsProspective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;31:840–845. PMID: 10733537
20. Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer. 2000;89:2266–2273. PMID: 11147597
21. Levy I, Sherman M. Staging of hepatocellular carcinoma: assessment of the CLIP, Okuda, and Child-Pugh staging systems in a cohort of 257 patients in Toronto. Gut. 2002;50:881–885. PMID: 12010894
22. Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, et al. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529–534. PMID: 11526539
23. Leung TW, Tang AM, Zee B, Yu SC, Lai PB, Lau WY, et al. Factors predicting response and survival in 149 patients with unresectable hepatocellular carcinoma treated by combination cisplatin, interferon-alpha, doxorubicin and 5-fluorouracil chemotherapy. Cancer. 2002;94:421–427. PMID: 11905412
24. Zhang XB, Wang JH, Yan ZP, Qian S, Du SS, Zeng ZC. Hepatocellular carcinoma with main portal vein tumor thrombus: treatment with 3-dimensional conformal radiotherapy after portal vein stenting and transarterial chemoembolization. Cancer. 2009;115:1245–1252. PMID: 19156918
25. Tan CK, Law NM, Ng HS, Machin D. Simple clinical prognostic model for hepatocellular carcinoma in developing countries and its validation. J Clin Oncol. 2003;21:2294–2298. PMID: 12805329
Fig. 1(A) Time to progression according to Eastern Cooperative Oncology Group (ECOG) performance status. (B) Overall survival in patients with and without portal vein thrombosis (PVT). 
Table 3Prognostic factors for time to disease progression and overall survival by univariate analysis 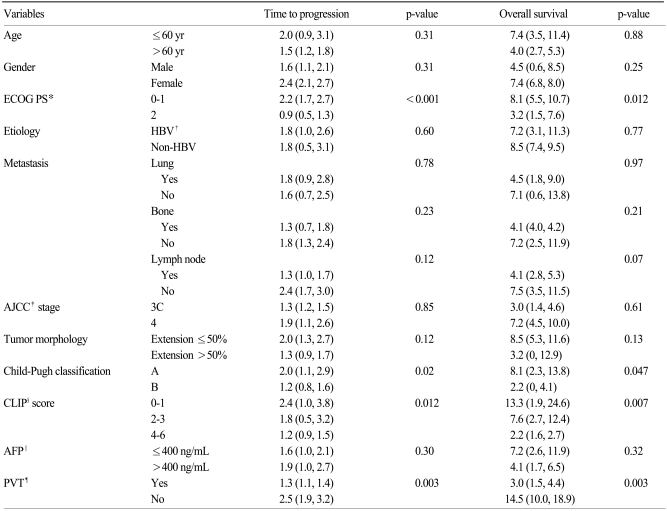
|
|