AbstractPurposeWe intend to evaluate the efficacy of salvage treatments for relapsed or refractory diffuse large B-cell lymphoma (R/R DLBCL) through meta-analysis.
Materials and MethodsR/R DLBCL trials were divided into two groups based on eligibility for autologous stem-cell transplantation (ASCT), and meta-analysis of each group was performed. Random effects models were used to estimate the 1-year progression-free survival (PFS) rate, and chimeric antigen receptor (CAR) T-cell therapy was used as reference treatment.
ResultsTwenty-six ASCT-eligible cohorts from 17 studies comprising 2,924 patients and 59 ASCT-ineligible cohorts from 53 studies comprising 3,617 patients were included in the pooled analysis. In the ASCT-eligible group, the pooled 1-year PFS rate was 0.40 (95% confidence interval [CI], 0.15 to 0.65) for the CAR T-cell group and 0.34 (95% CI, 0.30 to 0.37) for the group with chemotherapy followed by ASCT intention. The two treatments were not significantly different in meta-regression analysis. In the ASCT-ineligible group, the pooled 1-year PFS was 0.40 (95% CI, 0.35 to 0.46) for CAR T-cell, and the highest primary outcome was 0.47 (95% CI, 0.37 to 0.57) for the tafasitamab group. CAR T-cell therapy showed significantly better outcomes than chemotherapy and therapies based on ibrutinib, lenalidomide, and selinexor. However, loncastuximab, polatuzumab plus bendamustine and rituximab, and the tafasitamab group showed no different efficacy than CAR T-cell therapy after adjusting for median number of previous lines of treatment.
IntroductionDiffuse large B-cell lymphoma (DLBCL) is the globally most prevalent subtype of non-Hodgkin’s lymphoma, accounting for approximately 20% of all lymphoid malignancies [1]. Although the majority of patients with DLBCL responds to front-line R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy or a comparable regimen, 3%–40% of patients are refractory to the treatment or relapse after an initial response [2,3]. Standard treatment for patients with relapsed or refractory (R/R) DLBCL has been second-line chemotherapy followed by consolidative autologous stem-cell transplantation (ASCT). However, only a minority of R/R DLBCL patients can be cured by ASCT because the majority are ineligible due to old age, comorbidities, or refractory disease [4]. The prognosis of patients who are ineligible for ASCT or relapse even after ASCT remains dismal.
Chimeric antigen receptor (CAR) T-cell therapy targeting CD19 has advanced the treatment for R/R DLBCL patients and has achieved long-term remission in up to 40% of patients [5]. Additionally, recent randomized controlled trials that compared the efficacy of CAR T-cell therapy with the standard of care incorporating ASCT have suggested that CAR T-cell be considered as a second-line therapy for transplant-eligible patients who relapsed within 1 year after first-line treatment [6,7]. Nevertheless, CAR T-cell therapy has shown some limitations. For example, the therapy for later-line treatment showed a response in a limited population [5,8–10], and conflicting clinical outcomes have been reported for second-line treatment [6,7,11,12]. Other than CAR T-cell therapy, a variety of salvage treatments including novel therapeutic agents has been recommended in different situations depending on eligibility for ASCT, number of previous treatment lines, and cell of origin of the DLBCL [13]. In particular, since 2019, polatuzumab vedotin in combination with bendamustine and rituximab [14], selinexor [15], tafasitamab plus lenalidomide [16], and loncastuximab tesirine [17] have been approved by the FDA for reliable responses to R/R DLBCL.
Because it is challenging to analyze the efficacy of several recommended treatments and compare them through randomized trials, a pooled analysis of available studies and a comprehensive comparison of the various regimens could provide clinically relevant information. To reduce heterogeneity and analyze a population with similar characteristics, we divided all R/R DLBCL studies into two groups, one with ASCT-eligible patients and one with ASCT-ineligible patients and performed a meta-analysis in each group.
Materials and Methods1. Systematic literature reviewThis systematic review and meta-analysis were conducted according to the reporting guidelines in Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-Analysis of Observational Studies in Epidemiology [18]. The authors performed a comprehensive search of the literature in MEDLINE, Embase, and the Cochrane Central Register of Controlled Trial databases with language restriction in English from inception to 30 June 2022. The main keywords for the literature search were b-cell lymphoma and prospective phase II or III study, and the details of the search strategy are described in the supplementary Methods. Additionally, the meeting abstracts from the American Society of Clinical Oncology, American Society of Hematology, European Society for Medical Oncology, and European Hematology Association and the references of pertinent articles were manually scanned to identify additional relevant studies.
2. Eligibility criteriaProspective phase II or III trials evaluating the efficacy of systemic treatment for R/R DLBCL were selected for meta-analysis. Additional inclusion criteria were full-text articles and report of the primary outcome. Studies with the following characteristics were excluded: (1) no specific subtype outcome or characteristics of DLBCL among several types of B-cell lymphoma; (2) conducted before the first-line rituximab era; (3) mainly comprised patients with central nervous system lymphoma, b-cell cutaneous lymphoma, human immunodeficiency virus-related lymphoma, or primary mediastinal lymphoma; (4) studied the efficacy of radiotherapy only; (5) studied the efficacy of ASCT only; (6) had no information on previous treatments; and (7) studied a pediatric population.
3. Data extractionTwo authors independently extracted the following records from both ASCT-eligible and ineligible studies (J.K. and J.C.): trial identifier, published journal, publication year, name of the first author, trial phase, country where trial was conducted, treatment regimens, treatment category, and median follow-up duration. Clinical information of median age (range); median number of prior therapies (range); complete remission (CR) or partial remission (PR) rate; total number of patients; and proportions of patients with age ≥ 65 years, male gender, de novo DLBCL, germinal center B-cell like subtype, stage III/IV, Eastern Cooperative Oncology Group ≥ 2, and previous anti-CD20 treatment were also collected. In the ASCT-eligible studies, the proportion of patients with CR duration from front-line treatment (< or ≥ 12 months), who proceeded to primary refractoriness, high lactate dehydrogenase, International Prognostic Index (IPI) 4/5, second-line age-adjusted IPI 2/3, and ASCT were additionally collected. In the ASCT-ineligible studies, the percentage of prior ASCT, refractory to last treatment, and IPI 0–1/2–3/4–5 were obtained. The other two authors (S.E.Y. and S.J.K.) resolved discrepancies in the extracted data.
4. Classification of chemotherapyBased on current guidelines, classification of the chemotherapeutic agents for R/R DLBCL was performed [13]. In the group intending to proceed to ASCT, the regimen containing cytotoxic chemotherapy as the main backbone and attempting ASCT in responding patients was classified as chemotherapy, while CAR-T cell therapy was separately grouped. The regimens not recommended in the current guidelines were categorized as “others.” In the non-candidates for ASCT group, treatment consisting primarily of chemotherapy was classified as chemotherapy. Several targeted agents were classified into each category itself if specified in the guidelines. Accordingly, three treatment categories were generated in the ASCT-eligible group and nine in the ASCT-ineligible group. The regimens used in the ASCT-eligible studies were (1) CAR T-cell therapy, including axicabtagene ciloleucel [6], lisocabtagene maraleucel [7], and tisagenlecleucel [11]; (2) Chemotherapy, including ICD (irinotecan, cisplatin, and dexamethasone) [19], R (rituximab)-ICE (ifosfamide, carboplatin, and etoposide) [4,20], R-DHAP (cytarabine, cisplatin, and dexamethasone) [4,21,22], R-NIMP (vinorelbine, ifosfamide, mitoxantrone, and prednisolone) [23], ofatumumab plus DHAP [22,24], ofatumumab plus ICE [24], R-GDP (gemcitabine, dexamethasone, and cisplatin) [21,25], dacetuzumab plus R-ICE [20], R-OAD (oxaliplatin, cytarabine, and dexamethasone) [26], ofatumumab plus BCE (bendamustine, carboplatin, and etoposide) [27], temsirolimus plus R-DHAP [28], and platinum-based chemoimmunotherapy [6,7,11] used as a control in recent randomized trials that evaluated the efficacy of CAR T-cell therapy; and (3) others, including R-lenalidomide [29], inotuzumab ozogamicin [30], blinatumomab [31], and R-gemcitabine plus lenalidomide [25].
Treatments in the ASCT-ineligible studies were grouped as follows: (1) CAR T-cell therapy [5,32], including axicabtagene ciloleucel [8], lisocabtagene maraleucel [10], and tisagenlecleucel [9]; (2) chemotherapy, including R-Gemox (gemcitabine and oxaliplatin) [33,34], R-B (bendamustine) [14,35–38], pixantrone [39], one of the various chemotherapies [39] (one of vinorelbine, oxaliplatin, ifosfamide, etoposide, mitoxantrone, gemcitabine, or rituximab), R-ifosfamide+etoposide [40], R-pixantrone [41], R-gemcitabine [41], R-PECC (prednisolone, etoposide, chlorambucil, and lomustine) [42], decitabine plus DHAP [43], ibrutinib plus lenalidomide plus R-EPOCH (etoposide, prednisolone, vincristine, cyclophosphamide, and doxorubicin) [44], pixantrone plus obinutuzumab [45], and lenalidomide plus R-GDP [46]; (3) ibrutinib-based therapy, including ibrutinib plus durvalumab [47], ibrutinib plus nivolumab [48], and ibrutinib monotherapy [49]; (4) lenalidomide-based therapy, including R-lenalidomide [50], lenalidomide monotherapy [34], obinutuzumab plus lenalidomide [51], and temsirolimus plus lenalidomide [52]; (5) loncastuximab tesirine [17]; (6) polatuzumab plus bendamustine and rituximab [14,53,54] and R-polatuzumab [55]; (7) selinexor [15]; (8) tafasitamab, including tafasitamab monotherapy [56] and tafasitamab plus lenalidomide [16]; (9) others, including ibritumomab [57,58], temsirolimus [59], ofatumumab [60], R-everolimus [61], dacetuzumab [62], sepantronium [63], belinostat [64], R-coltuximab [65], R-panobinostat [66], mocetinostat [67], buparlisib [68], abexinostat [69], coltuximab [70], entospletinib [71], panobinostat [72,73], R-inotuzumab [37], selumetinib [74], nivolumab [75], R-pinatuzumab [55], and copanlisib [76].
5. Data synthesis and analysesThe primary outcome was 1-year progression-free survival (PFS) rate, and the secondary outcome was CR rate according to treatment category. There are two main reasons why 1-year PFS rate was used as a primary endpoint; First, disease progression takes place mostly within the first year and thereafter tends to form a plateau with most treatments, including CAR T-cell therapy, in most included trials. Second, most of the included studies reported 1-year PFS rate, event-free survival rate, or related Kaplan-Meier survival graphs. When the 1-year PFS rate was only demonstrated as a Kaplan-Meier survival graph, the software GetData Graph Digitizer 2.26 (http://getdata-graph-digitizer.com/) was used for digitizing and extracting data. The pooled primary and secondary outcomes and corresponding 95% confidence intervals (CIs) and p-values were calculated using random-effects models. Our model was a single-arm proportion meta-analysis, and the calculations were performed using the inverse variance method. To assess 1-year PFS and the CR rate according to treatment category, subgroup analyses using a Q test were performed. In addition, a meta-regression was conducted to evaluate potential moderators associated with the primary outcome. The patient proportions of stage III/IV, duration of complete response greater or less than 12 months from front-line treatment, and primary refractoriness to previous therapy were included in the ASCT-eligible studies. The proportions of stage III/IV, prior ASCT, refractoriness to the last treatment, and median number of prior lines of treatment were included in the ASCT-ineligible studies. The variables with p-value of < 0.1 in univariate meta-regression or predefined variables with clinical significance, especially the percentage of primary refractoriness to previous therapy in the ASCT-eligible group, were incorporated in the multivariate meta-regression analysis. Meta-regression was executed using the metareg function with its default parameters and a mixed-effects model. Heterogeneity was assessed using the τ2 and I2 statistics. All statistical tests were two-sided, and p-values ≤ 0.05 were regarded as statistically significant. R studio software (ver. 1.4.1743, R Foundation for Statistical Computing, Vienna, Austria) was utilized for quantitative pooled analysis and meta-regression modeling.
Results1. Literature searchA total of 9,670 records was identified during the initial database search. After removing duplicates and screening the titles and abstracts of the studies, 811 potentially relevant studies remained for detailed review. As described above, additional exclusion criteria were applied to derive the final 17 studies [4,6,7,11,19–31] comprising 26 cohorts and 2,924 ASCT-eligible patients and 53 studies [5,8,9,10,14–17,32–76] of 59 cohorts and 3,617 ASCT-ineligible patients included in the meta-analysis (Fig. 1).
2. Characteristics of included studiesThe baseline characteristics of the 26 ASCT-eligible cohorts are outlined in Table 1 and S1 Table, and the baseline characteristics of the 59 ASCT-ineligible cohorts are outlined in Table 2 and S2 Table. In the ASCT-eligible studies, six studies including 12 cohorts were phase 3 randomized trials and were published in 2008–2022. The categories of regimens were classified as follows: 19 cohorts from 14 studies [4,6,7,11,19–28] with chemotherapy; three cohorts from three studies [6,7,11] with CAR T-cell therapy; and four cohorts from four studies [25,29–31] with others. Only one cohort [29] reported a median number of prior systemic therapies of 3, while all others reported a median number of one prior systemic therapy. Studies comparing CAR T-cell therapy and chemotherapy [6,7,11] in randomized controlled trials had no patients who maintained CR for more than 12 months from front-line treatment. All but two cohorts [24] and cohorts treated with CAR T-cell reported various ranges of proportions of patients to proceed to ASCT. In ASCT-ineligible studies, 10 cohorts from five studies [34,37,39,41,55] were randomized trials and were published in 2007–2022. The following nine categories were used to classify regimens: five cohorts from five studies [5,8-10,32] with CAR T-cell therapy; 17 cohorts from 16 studies [14,33–46,61] with chemotherapy; three cohorts from three studies [47–49] with ibrutinib-based therapy; four cohorts from four studies [34,50–52] with lenalidomide-based therapy; one cohort from one study [17] with loncastuximab; four cohorts from four studies [14,53–55] with polatuzumab plus bendamustine and rituximab; one cohort from one study [15] with selinexor; two cohorts from two studies [16,56] with tafasitamab; and 22 cohorts from 21 [37,55,57–60,62–76] studies with others. The median number of previous treatments ranged from 1 to 5, and all CAR T-cell studies reported a median of three previous treatments.
3. Pooled analyses and meta-regression–ASCT-eligible trialsThe overall pooled proportion for 1-year PFS in ASCT-eligible studies was 0.33 (95% CI, 0.28 to 0.38). There were no statistically significant differences in 1-year PFS by category (p=0.30); 0.40 (95% CI, 0.15 to 0.65) in the CAR T-cell therapy group, 0.34 (95% CI, 0.30 to 0.37) in the chemotherapy group, and 0.21 (95% CI, 0.05 to 0.38) in others (Fig. 2). Meta-regression analysis with a mixed-effects model using predefined and relevant study-level moderators was conducted to evaluate whether several factors influenced the outcome (Table 3). Univariate meta-regression revealed that proportion of stage III/IV patients, duration of complete response greater or less than 12 months from front-line treatment, and primary refractoriness to previous therapy were not significantly associated with 1-year PFS rate. The only factor associated with the primary outcome was the CR rate for testing the performance of the regression analysis. According to the predefined analysis principle, primary refractoriness to prior treatment was included in the multivariable meta-regression model, and there was still no significant difference between the Chemotherapy and CAR T-cell groups. The pooled CR rate was 0.31 (95% CI, 0.25 to 0.36), and significantly different CR rates were observed according to regimen (p=0.01) (S3 Fig.). CR rate was 0.53 (95% CI, 0.29 to 0.78) for the CAR T-cell therapy group, 0.28 (95% CI, 0.23 to 0.33) for the Chemotherapy group, and 0.20 (95% CI, 0.13 to 0.27) for the others group. The pooled CR rate of CAR T-cell therapy was significantly higher than those of the Chemotherapy and Others groups.
4. Pooled analyses and meta-regression – ASCT-ineligible trialsThe overall pooled 1-year PFS rate in ASCT-ineligible trials was 0.24 (95% CI, 0.20 to 0.27). The significant differences in 1-year PFS according to category (p < 0.01) were 0.40 (95% CI, 0.35 to 0.46) for CAR T-cell therapy, 0.23 (95% CI, 0.17 to 0.30) for chemotherapy, 0.24 (95% CI, 0.14 to 0.34) for ibrutinib-based therapy, 0.26 (95% CI, 0.18 to 0.35) for lenalidomide-based therapy, 0.26 (95% CI, 0.19 to 0.3,3) for loncastuximab, 0.35 (95% CI, 0.27 to 0.42) for polatuzumab plus bendamustine and rituximab, 0.21 (95% CI, 0.15 to 0.29) for selinexor, 0.47 (95% CI, 0.37 to 0.57) for tafasitamab, and 0.16 (95% CI, 0.11 to 0.21) for others (Fig. 3). Meta-regression analysis demonstrated that patient proportion of stage III/IV, prior ASCT, and refractoriness to the last treatment had no association with the outcome in univariate meta-regression. The median number of prior lines of treatment showed a weak correlation with 1-year PFS rate and was included in the multivariable meta-regression model with regimen. This model revealed that CAR T-cell therapy was significantly better than chemotherapy, ibrutinib-based therapy, lenalidomide-based therapy, and selinexor but showed no difference in efficacy compared to loncastuximab, polatuzumab plus bendamustine and rituximab, and tafasitamab, with adjustment for median number of previous lines of treatment (Table 4). The pooled proportion of CR was 0.20 (95% CI, 0.16 to 0.24) (S4 Fig.), and CAR T-cell therapy (0.45; 95% CI, 0.37 to 0.54) demonstrated a better CR rate than all other groups except loncastuximab and polatuzumab plus bendamustine and rituximab (data not shown).
DiscussionThis meta-analysis has focused on investigating the relationships between various treatment regimens and PFS outcomes in R/R DLBCL patients. Although CAR T-cell therapy has emerged as a promising therapeutic strategy, relevant trials have reported conflicting results compared to the standard treatment in ASCT-eligible patients. In addition, no direct evidence has demonstrated whether CAR T-cell therapy is superior to other recommended treatments in the ASCT-ineligible group. In this study, we evaluated the efficacy of different regimens using a single-arm proportion meta-analysis and meta-regression according to eligibility for ASCT, using CAR T-cell therapy as a reference treatment for comparison. In the studies examined, 26 cohorts from 17 prospective ASCT-eligible studies and 59 cohorts from 53 ASCT-ineligible studies were included. CAR T-cell treatment did not show a significantly better 1-year PFS rate than chemotherapy in the former group and did not demonstrate superior outcomes compared to loncastuximab, polatuzumab plus bendamustine and rituximab, or tafasitamab in the latter group.
The impressive results of anti-CD19 CAR T cells as a third-line therapy prompted testing as second-line treatment for refractory DLBCL patients eligible for ASCT, but the use of these therapies in this population is a current subject of debate. Three large randomized phase III studies, the ZUMA-7 [6], BELINDA [11], and TRANSFORM [7] trials, all of which included patients with primary refractory disease or with relapse within 12 months of completion of front-line therapy, were conducted by comparing the 3 CAR T-cell products and salvage platinum-based chemotherapy followed by ASCT. The ZUMA-7 and TRANSFORM trial demonstrated the superiority of each CAR T-cell therapy over standard treatment in terms of event-free survival, but no difference between CAR T and chemotherapy was observed in the BELINDA trial. Substantial factors such as study designs in the 3 trials, including use and type of bridging therapy, percentage of patients who received bridging therapy, and proportion of patients who had progressive disease before CAR T-cell infusion, might explain these conflicting clinical outcomes [77].
These heterogeneities in baseline features within the CAR T-cell trials and within studies included in the ASCT-eligible group led to the unexpected result of our pooled analysis. To reduce heterogeneity, the proportion of patients who were primarily refractory to front-line treatment was used as the adjustment factor because a strong association between the variable and 1-year PFS rate was identified in the chemotherapy category group (p=0.001, data not shown) in our meta-regression model. Nonetheless, the difference in primary outcome between the CAR T-cell and the chemotherapy followed by ASCT group was not significant in ASCT-eligible analysis.
A recent meta-analysis by Shargian et al. [78], in which the ZUMA-7 [6], BELINDA [11], and TRANSFORM [7] trials were pooled for analysis, has demonstrated that clinical outcomes were significantly improved with CAR T compared to the standard of care of second-line treatment [78]. However, the number of studies included in the meta-analysis was small, and the heterogeneity between studies due to the variability in the design of the studies was highly reflected. In contrast, a recent CIBMTR-comparative analysis involving 411 patients who achieved at least PR from salvage therapy has shown support for the use of ASCT among chemosensitive patients when CAR T-cell treatment was also available [79]. The 2-year PFS was comparable between those who received ASCT versus those who received CAR T-cell, and a lower risk of relapse as well as improved 2-year overall survival were reported in the ASCT cohort. Therefore, considering the advantages of ASCT over CAR T-cells, such as low toxicity, inferior cost, and broader access for patients, careful selection and use of high-dose chemotherapy for patients with the chemosensitive disease is thought to be reasonable. Prospective trials for non-high-risk aggressive B-cell lymphomas and the definition of an optimal strategy for bridging therapy are needed.
Several studies have compared matched populations of CAR T-cell trial and historical salvage chemotherapy trial that mainly comprised ASCT-ineligible patients using propensity score-matched analysis [80,81]. All CAR T-cell therapies demonstrated durable responses and survival benefits over chemotherapy for patients with refractory DLBCL. In our pooled analysis, the CAR T-cell therapy group showed significantly better 1-year PFS than the Chemotherapy group, in line with previous findings. However, comparing CAR T-cell therapy and recently approved regimens, including loncastuximab, polatuzumab plus bendamustine and rituximab, and tafasitamab, no significant different primary outcomes were identified in our study. Among these new agents, in particular, the efficacy of the tafasitamab plus lenalidomide regimen from the L-MIND trial [16] was compared with matched-paired patients treated with recommended therapies for ASCT-ineligible R/R DLBCL in an observational retrospective study [82]. This regimen showed better overall survival and overall response compared to polatuzumab plus bendamustine/rituximab and rituximab plus lenalidomide and comparable outcomes to CAR T-cells, highlighting its prominent efficacy despite the limitation of the retrospective nature. These findings indicate that there will be roles of newly developed targeted agents other than CAR T-cell therapy in heavily treated patients and those ineligible for ASCT.
There are some inherent limitations in the current study. First, we were unable to thoroughly analyze patient-level covariates due to the study’s characteristic of a study-level meta-analysis of a prospective single-arm study. Similarly, determinations for ASCT eligibility were based on the study design rather than individual characteristics. Second, it was difficult to evaluate the efficacy of each regimen because the several treatment regimens were crudely grouped for classification and analysis. Third, in the ASCT-ineligible group analysis, multiple comparisons were unavoidable because an increased number of treatment groups was generated according to the current guidelines. Despite these limitations, our meta-analysis was carried out using solid statistical methodologies with a strict set of inclusion criteria, allowing us to confirm the relative efficacy of CAR T-cell therapy compared to several other treatments.
In summary, this pooled analysis revealed that CAR T-cell therapy did not show a significantly superior 1-year PFS rate over ASCT following chemotherapy in a second-line setting. In addition, CAR T-cell did not outperform several recently developed agents in patients who were ineligible for ASCT. Given the limited number of relevant trials available for each treatment category group and their heterogeneity, further well-conducted large-scale studies are required to corroborate our findings.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesFig. 1Trial selection flow. ASCT, autologous stem cell transplantation; HIV, human immunodeficiency virus. 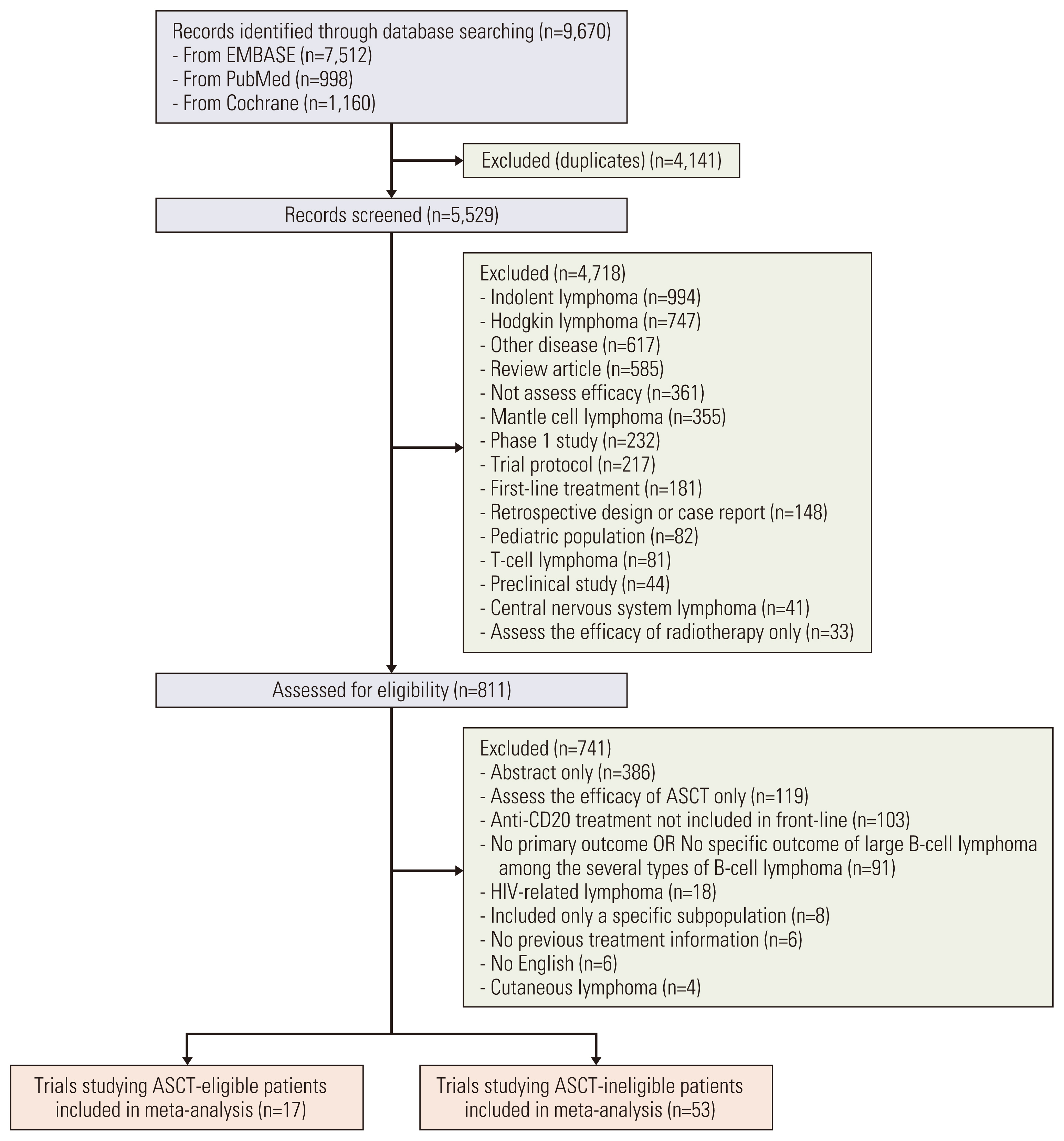
Table 1Baseline characteristics of trials studying ASCT-eligible patients
ASCT, autologous stem cell transplantation; BCE, bendamustine, carboplatin, and etoposide; CAR, chimeric antigen receptor; DHAP, cytarabine, cisplatin, and dexamethasone; GDP, gemcitabine, dexamethasone, and cisplatin; ICD, irinotecan, cisplatin, and dexamethasone; IQR, interquartile range; NA, not available; NIMP, vinorelbine, ifosfamide, mitoxantrone, and prednisolone; OAD, oxaliplatin, cytarabine, and dexamethasone; R-ICE, rituximab-ifosfamide, carboplatin, and etoposide. Table 2Baseline characteristics of trials studying ASCT-ineligible patients
ASCT, autologous stem cell transplantation; B, bendamustine; CAR, chimeric antigen receptor; DHAP, cytarabine, cisplatin, and dexamethasone; EPOCH, etoposide, predni-solone, vincristine, cyclophosphamide, and doxorubicin; GDP, gemcitabine, dexamethasone, and cisplatin; IQR, interquartile range; NA, not available; PECC, prednisolone, etoposide, chlorambucil, and lomustine; R-GemOx, rituximab-gemcitabine and oxaliplatin. Table 3Meta-regression analysis using study-level characteristics in relation to 1-year PFS rate in ASCT-eligible studies
Table 4Meta-regression analysis using study-level characteristics in relation to 1-year PFS rate in ASCT-ineligible studies References1. Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66:443–59.
3. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116:2040–5.
4. Gisselbrecht C, Glass B, Mounier N, Singh Gill D, Linch DC, Trneny M, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28:4184–90.
5. Schuster SJ, Svoboda J, Chong EA, Nasta SD, Mato AR, Anak O, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–54.
6. Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386:640–54.
7. Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399:2294–308.
8. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–44.
9. Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56.
10. Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396:839–52.
11. Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022;386:629–39.
12. Roschewski M, Longo DL, Wilson WH. CAR T-cell therapy for large B-cell lymphoma: who, when, and how? N Engl J Med. 2022;386:692–6.
13. National Comprehensive Cancer Network. NCCN clinical practice guidelines: B-cell lymphomas V5.2022 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2022. [cited 2022 Aug 7]. Available from: https://www.nccn.org/professionals/physcian_gls/pdf/b-cell.pdf
14. Sehn LH, Herrera AF, Flowers CR, Kamdar MK, McMillan A, Hertzberg M, et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2020;38:155–65.
15. Kalakonda N, Maerevoet M, Cavallo F, Follows G, Goy A, Vermaat JS, et al. Selinexor in patients with relapsed or refractory diffuse large B-cell lymphoma (SADAL): a single-arm, multinational, multicentre, open-label, phase 2 trial. Lancet Haematol. 2020;7:e511–22.
16. Salles G, Duell J, Gonzalez Barca E, Tournilhac O, Jurczak W, Liberati AM, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21:978–88.
17. Caimi PF, Ai W, Alderuccio JP, Ardeshna KM, Hamadani M, Hess B, et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2021;22:790–800.
18. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12.
19. Kang HJ, Kim WS, Suh C, Park YH, Kim BS, Yuh YJ, et al. Irinotecan plus cisplatin and dexamethasone (ICD) combination chemotherapy for patients with diffuse large B-cell lymphoma previously treated with rituximab plus CHOP. Cancer Chemother Pharmacol. 2008;62:299–304.
20. Fayad L, Ansell SM, Advani R, Coiffier B, Stuart R, Bartlett NL, et al. Dacetuzumab plus rituximab, ifosfamide, carboplatin and etoposide as salvage therapy for patients with diffuse large B-cell lymphoma relapsing after rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone: a randomized, double-blind, placebo-controlled phase 2b trial. Leuk Lymphoma. 2015;56:2569–78.
21. Crump M, Kuruvilla J, Couban S, MacDonald DA, Kukreti V, Kouroukis CT, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem-cell transplantation for relapsed and refractory aggressive lymphomas: NCIC-CTG LY.12. J Clin Oncol. 2014;32:3490–6.
22. van Imhoff GW, McMillan A, Matasar MJ, Radford J, Ardeshna KM, Kuliczkowski K, et al. Ofatumumab versus rituximab salvage chemoimmunotherapy in relapsed or refractory diffuse large B-cell lymphoma: the ORCHARRD study. J Clin Oncol. 2017;35:544–51.
23. Gyan E, Damotte D, Courby S, Senecal D, Quittet P, Schmidt-Tanguy A, et al. High response rate and acceptable toxicity of a combination of rituximab, vinorelbine, ifosfamide, mito-xantrone and prednisone for the treatment of diffuse large B-cell lymphoma in first relapse: results of the R-NIMP GOELAMS study. Br J Haematol. 2013;162:240–9.
24. Matasar MJ, Czuczman MS, Rodriguez MA, Fennessy M, Shea TC, Spitzer G, et al. Ofatumumab in combination with ICE or DHAP chemotherapy in relapsed or refractory intermediate grade B-cell lymphoma. Blood. 2013;122:499–506.
25. Kuhnl A, Peckitt C, Patel B, Ardeshna KM, Macheta MP, Radford J, et al. R-GEM-Lenalidomide versus R-GEM-P as second-line treatment of diffuse large B-cell lymphoma: results of the UK NRCI phase II randomised LEGEND trial. Ann Hematol. 2020;99:105–12.
26. Witzig TE, Johnston PB, LaPlant BR, Kurtin PJ, Pederson LD, Moore DF Jr, et al. Long-term follow-up of chemoimmunotherapy with rituximab, oxaliplatin, cytosine arabinoside, dexamethasone (ROAD) in patients with relapsed CD20+ B-cell non-Hodgkin lymphoma: results of a study of the Mayo Clinic Cancer Center Research Consortium (MCCRC) MC-0485 now known as academic and community cancer research united (ACCRU). Am J Hematol. 2017;92:1004–10.
27. Pan J, Ghimire S, Alpdogan SO, Chapman A, Carabasi M, DiMeglio M, et al. Phase I/II study of bendamustine in combination with ofatumumab, carboplatin, etoposide (BOCE) for relapsed or refractory aggressive B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2021;62:590–7.
28. Witzens-Harig M, Viardot A, Keller U, Wosniok J, Deuster O, Klemmer J, et al. The mTOR inhibitor temsirolimus added to rituximab combined with dexamethasone, cytarabine, and cisplatinum (R-DHAP) for the treatment of patients with relapsed or refractory DLBCL: results from the phase-II STORM trial. Hemasphere. 2021;5:e636.
29. Wang M, Fowler N, Wagner-Bartak N, Feng L, Romaguera J, Neelapu SS, et al. Oral lenalidomide with rituximab in relapsed or refractory diffuse large cell, follicular and transformed lymphoma: a phase II clinical trial. Leukemia. 2013;27:1902–9.
30. Wagner-Johnston ND, Goy A, Rodriguez MA, Ehmann WC, Hamlin PA, Radford J, et al. A phase 2 study of inotuzumab ozogamicin and rituximab, followed by autologous stem cell transplant in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma. 2015;56:2863–9.
31. Coyle L, Morley NJ, Rambaldi A, Mason KD, Verhoef G, Furness CL, et al. Open-label, phase 2 study of blinatumomab as second salvage therapy in adults with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Leuk Lymphoma. 2020;61:2103–12.
32. Sang W, Shi M, Yang J, Cao J, Xu L, Yan D, et al. Phase II trial of co-administration of CD19- and CD20-targeted chimeric antigen receptor T cells for relapsed and refractory diffuse large B cell lymphoma. Cancer Med. 2020;9:5827–38.
33. Lopez A, Gutierrez A, Palacios A, Blancas I, Navarrete M, Morey M, et al. GEMOX-R regimen is a highly effective salvage regimen in patients with refractory/relapsing diffuse large-cell lymphoma: a phase II study. Eur J Haematol. 2008;80:127–32.
34. Czuczman MS, Trneny M, Davies A, Rule S, Linton KM, Wagner-Johnston N, et al. A phase 2/3 multicenter, randomized, open-lbel study to compare the efficacy and safety of lenalidomide versus investigator’s choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res. 2017;23:4127–37.
35. Ohmachi K, Niitsu N, Uchida T, Kim SJ, Ando K, Takahashi N, et al. Multicenter phase II study of bendamustine plus rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol. 2013;31:2103–9.
36. Vacirca JL, Acs PI, Tabbara IA, Rosen PJ, Lee P, Lynam E. Bendamustine combined with rituximab for patients with relapsed or refractory diffuse large B cell lymphoma. Ann Hematol. 2014;93:403–9.
37. Dang NH, Ogura M, Castaigne S, Fayad LE, Jerkeman M, Radford J, et al. Randomized, phase 3 trial of inotuzumab ozogamicin plus rituximab versus chemotherapy plus rituximab for relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Br J Haematol. 2018;182:583–6.
38. Murayama K, Kiguchi T, Izutsu K, Kameoka Y, Hidaka M, Kato H, et al. Bendamustine plus rituximab in Japanese patients with relapsed or refractory diffuse large B-cell lymphoma. Ann Hematol. 2022;101:979–89.
39. Pettengell R, Sebban C, Zinzani PL, Derigs HG, Kravchenko S, Singer JW, et al. Monotherapy with pixantrone in histologically confirmed relapsed or refractory aggressive B-cell non-Hodgkin lymphoma: post-hoc analyses from a phase III trial. Br J Haematol. 2016;174:692–9.
40. Joshi M, Taper J, Forsyth C, Rowlings P, Campbell P, Crispin P, et al. Outpatient rituximab, ifosfamide, etoposide (R-IE) in patients older than 60 years with relapsed or refractory diffuse large B-cell lymphoma who are not candidates for stem cell transplantation. Leuk Lymphoma. 2020;61:91–7.
41. Pettengell R, Dlugosz-Danecka M, Andorsky D, Belada D, Georgiev P, Quick D, et al. Pixantrone plus rituximab versus gemcitabine plus rituximab in patients with relapsed aggressive B-cell non-Hodgkin lymphoma not eligible for stem cell transplantation: a phase 3, randomized, multicentre trial (PIX306). Br J Haematol. 2020;188:240–8.
42. Lugtenburg PJ, Zijlstra JM, Doorduijn JK, Bohmer LH, Hoogendoorn M, Berenschot HW, et al. Rituximab-PECC induction followed by (90) Y-ibritumomab tiuxetan consolidation in relapsed or refractory DLBCL patients who are ineligible for or have failed ASCT: results from a phase II HOVON study. Br J Haematol. 2019;187:347–55.
43. Hu J, Wang X, Chen F, Ding M, Dong M, Yang W, et al. Combination of decitabine and a modified regimen of cisplatin, cytarabine and dexamethasone: a potential salvage regimen for relapsed or refractory diffuse large B-cell lymphoma after second-line treatment failure. Front Oncol. 2021;11:687374.
44. Wilson WH, Phillips T, Popplewell L, de Vos S, Chhabra S, Kimball AS, et al. Phase 1b/2 study of ibrutinib and lenalidomide with dose-adjusted EPOCH-R in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma. 2021;62:2094–106.
45. Hess G, Huttmann A, Witzens-Harig M, Dreyling MH, Keller U, Marks R, et al. A phase II trial to evaluate the combination of pixantrone and obinutuzumab for patients with relapsed aggressive lymphoma: final results of the prospective, multicentre GOAL trial. Br J Haematol. 2022;198:482–91.
46. Palazon-Carrion N, Martin Garcia-Sancho A, Nogales-Fer-nandez E, Jimenez-Cortegana C, Carnicero-Gonzalez F, Rios-Herranz E, et al. Lenalidomide plus R-GDP (R2-GDP) in relapsed/refractory diffuse large B-cell lymphoma: final results of the R2-GDP-GOTEL trial and immune biomarker subanalysis. Clin Cancer Res. 2022;28:3658–68.
47. Herrera AF, Goy A, Mehta A, Ramchandren R, Pagel JM, Svoboda J, et al. Safety and activity of ibrutinib in combination with durvalumab in patients with relapsed or refractory follicular lymphoma or diffuse large B-cell lymphoma. Am J Hematol. 2020;95:18–27.
48. Younes A, Brody J, Carpio C, Lopez-Guillermo A, Ben-Yehuda D, Ferhanoglu B, et al. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019;6:e67–78.
49. Graf SA, Cassaday RD, Morris K, Voutsinas JM, Wu QV, Behnia S, et al. Ibrutinib monotherapy in relapsed or refractory, transformed diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2021;21:176–81.
50. Zinzani PL, Pellegrini C, Gandolfi L, Stefoni V, Quirini F, Derenzini E, et al. Combination of lenalidomide and rituximab in elderly patients with relapsed or refractory diffuse large B-cell lymphoma: a phase 2 trial. Clin Lymphoma Myeloma Leuk. 2011;11:462–6.
51. Houot R, Cartron G, Bijou F, de Guibert S, Salles GA, Fruchart C, et al. Obinutuzumab plus Lenalidomide (GALEN) for the treatment of relapse/refractory aggressive lymphoma: a phase II LYSA study. Leukemia. 2019;33:776–80.
52. Major A, Kline J, Karrison TG, Fishkin PA, Kimball AS, Petrich AM, et al. Phase I/II clinical trial of temsirolimus and lenalidomide in patients with relapsed and refractory lymphomas. Haematologica. 2022;107:1608–18.
53. Terui Y, Rai S, Izutsu K, Yamaguchi M, Takizawa J, Kuroda J, et al. A phase 2 study of polatuzumab vedotin+bendamustine +rituximab in relapsed/refractory diffuse large B-cell lymphoma. Cancer Sci. 2021;112:2845–54.
54. Sehn LH, Hertzberg M, Opat S, Herrera AF, Assouline S, Flowers CR, et al. Polatuzumab vedotin plus bendamustine and rituximab in relapsed/refractory DLBCL: survival update and new extension cohort data. Blood Adv. 2022;6:533–43.
55. Morschhauser F, Flinn IW, Advani R, Sehn LH, Diefenbach C, Kolibaba K, et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol. 2019;6:e254–65.
56. Jurczak W, Zinzani PL, Gaidano G, Goy A, Provencio M, Nagy Z, et al. Phase IIa study of the CD19 antibody MOR208 in patients with relapsed or refractory B-cell non-Hodgkin’s lymphoma. Ann Oncol. 2018;29:1266–72.
57. Morschhauser F, Illidge T, Huglo D, Martinelli G, Paganelli G, Zinzani PL, et al. Efficacy and safety of yttrium-90 ibritumomab tiuxetan in patients with relapsed or refractory diffuse large B-cell lymphoma not appropriate for autologous stem-cell transplantation. Blood. 2007;110:54–8.
58. Arnason JE, Luptakova K, Rosenblatt J, Tzachanis D, Avigan D, Zwicker JI, et al. Yttrium-90 ibritumomab tiuxetan followed by rituximab maintenance as treatment for patients with diffuse large B-cell lymphoma are not candidates for autologous stem cell transplant. Acta Haematol. 2015;133:347–53.
59. Smith SM, van Besien K, Karrison T, Dancey J, McLaughlin P, Younes A, et al. Temsirolimus has activity in non-mantle cell non-Hodgkin’s lymphoma subtypes: the University of Chicago phase II consortium. J Clin Oncol. 2010;28:4740–6.
60. Coiffier B, Radford J, Bosly A, Martinelli G, Verhoef G, Barca G, et al. A multicentre, phase II trial of ofatumumab monotherapy in relapsed/progressive diffuse large B-cell lymphoma. Br J Haematol. 2013;163:334–42.
61. Barnes JA, Jacobsen E, Feng Y, Freedman A, Hochberg EP, LaCasce AS, et al. Everolimus in combination with rituximab induces complete responses in heavily pretreated diffuse large B-cell lymphoma. Haematologica. 2013;98:615–9.
62. de Vos S, Forero-Torres A, Ansell SM, Kahl B, Cheson BD, Bartlett NL, et al. A phase II study of dacetuzumab (SGN-40) in patients with relapsed diffuse large B-cell lymphoma (DLBCL) and correlative analyses of patient-specific factors. J Hematol Oncol. 2014;7:44.
63. Papadopoulos KP, Lopez-Jimenez J, Smith SE, Steinberg J, Keating A, Sasse C, et al. A multicenter phase II study of sepantronium bromide (YM155) plus rituximab in patients with relapsed aggressive B-cell Non-Hodgkin lymphoma. Leuk Lymphoma. 2016;57:1848–55.
64. Puvvada SD, Li H, Rimsza LM, Bernstein SH, Fisher RI, LeBlanc M, et al. A phase II study of belinostat (PXD101) in relapsed and refractory aggressive B-cell lymphomas: SWOG S0520. Leuk Lymphoma. 2016;57:2359–69.
65. Coiffier B, Thieblemont C, de Guibert S, Dupuis J, Ribrag V, Bouabdallah R, et al. A phase II, single-arm, multicentre study of coltuximab ravtansine (SAR3419) and rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. Br J Haematol. 2016;173:722–30.
66. Assouline SE, Nielsen TH, Yu S, Alcaide M, Chong L, MacDonald D, et al. Phase 2 study of panobinostat with or without rituximab in relapsed diffuse large B-cell lymphoma. Blood. 2016;128:185–94.
67. Batlevi CL, Crump M, Andreadis C, Rizzieri D, Assouline SE, Fox S, et al. A phase 2 study of mocetinostat, a histone deacetylase inhibitor, in relapsed or refractory lymphoma. Br J Haematol. 2017;178:434–41.
68. Younes A, Salles G, Martinelli G, Bociek RG, Barrigon DC, Barca EG, et al. Pan-phosphatidylinositol 3-kinase inhibition with buparlisib in patients with relapsed or refractory non-Hodgkin lymphoma. Haematologica. 2017;102:2104–12.
69. Ribrag V, Kim WS, Bouabdallah R, Lim ST, Coiffier B, Illes A, et al. Safety and efficacy of abexinostat, a pan-histone deacetylase inhibitor, in non-Hodgkin lymphoma and chronic lymphocytic leukemia: results of a phase II study. Haematologica. 2017;102:903–9.
70. Trneny M, Verhoef G, Dyer MJ, Ben Yehuda D, Patti C, Canales M, et al. A phase II multicenter study of the anti-CD19 antibody drug conjugate coltuximab ravtansine (SAR3419) in patients with relapsed or refractory diffuse large B-cell lymphoma previously treated with rituximab-based immunotherapy. Haematologica. 2018;103:1351–8.
71. Burke JM, Shustov A, Essell J, Patel-Donnelly D, Yang J, Chen R, et al. An open-label, phase II trial of entospletinib (GS-9973), a selective spleen tyrosine kinase inhibitor, in diffuse large B-cell lymphoma. Clin Lymphoma Myeloma Leuk. 2018;18:e327–31.
72. Barnes JA, Redd R, Fisher DC, Hochberg EP, Takvorian T, Neuberg D, et al. Panobinostat in combination with rituximab in heavily pretreated diffuse large B-cell lymphoma: results of a phase II study. Hematol Oncol. 2018;36:633–7.
73. Zaja F, Salvi F, Rossi M, Sabattini E, Evangelista A, Ciccone G, et al. Single-agent panobinostat for relapsed/refractory diffuse large B-cell lymphoma: clinical outcome and correlation with genomic data: a phase 2 study of the Fondazione Italiana Linfomi. Leuk Lymphoma. 2018;59:2904–10.
74. Galanina N, Smith SM, Liao C, Petrich A, Libao B, Gartenhaus R, et al. University of Chicago phase II consortium trial of selumetinib (MEKi) demonstrates low tolerability and efficacy in relapsed DLBCL. Br J Haematol. 2018;181:264–7.
75. Ansell SM, Minnema MC, Johnson P, Timmerman JM, Armand P, Shipp MA, et al. Nivolumab for relapsed/refractory diffuse large B-cell lymphoma in patients ineligible for or having failed autologous transplantation: a single-arm, phase II study. J Clin Oncol. 2019;37:481–9.
76. Lenz G, Hawkes E, Verhoef G, Haioun C, Thye Lim S, Seog Heo D, et al. Single-agent activity of phosphatidylinositol 3-kinase inhibition with copanlisib in patients with molecularly defined relapsed or refractory diffuse large B-cell lymphoma. Leukemia. 2020;34:2184–97.
78. Shargian L, Raanani P, Yeshurun M, Gafter-Gvili A, Gurion R. Chimeric antigen receptor T-cell therapy is superior to standard of care as second-line therapy for large B-cell lymphoma: A systematic review and meta-analysis. Br J Haematol. 2022;198:838–46.
79. Shadman M, Pasquini M, Ahn KW, Chen Y, Turtle CJ, Hematti P, et al. Autologous transplant vs chimeric antigen receptor T-cell therapy for relapsed DLBCL in partial remission. Blood. 2022;139:1330–9.
80. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Reagan PM, Miklos DB, et al. Comparison of 2-year outcomes with CAR T cells (ZUMA-1) vs salvage chemotherapy in refractory large B-cell lymphoma. Blood Adv. 2021;5:4149–55.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||