AbstractPurposePopulation-based comparisons between minimally invasive surgery (MIS) (robotic surgery [RS] and laparoscopic surgery [LS]) and open surgery (OS) for managing endometrial cancer are lacking. This study aimed to compare surgical and oncologic outcomes between endometrial cancer patients who underwent surgical staging via MIS or OS.
Materials and MethodsA population-based retrospective cohort study was performed using claims data from the Korean National Health Insurance database from January 2012 to December 2016. All patients who underwent hysterectomy under diagnosis of endometrial cancer were identified. Patients were classified into RS, LS, and OS groups. Operative and oncologic outcomes were compared among the three groups after adjustments for age group, risk group (adjuvant therapy status), modified Charlson comorbidity index, income level, insurance type, and index year using propensity scores obtained via the inverse probability of treatment weighted method.
ResultsAfter adjustment, 5,065 patients (RS, n=315; LS, n=3,248; OS, n=1,503) were analyzed. Patient demographics were comparable. Hospital stay, postoperative complications, and cost were more favorable in the RS and LS groups than in the OS group (all p < 0.001). Five-year overall survival was significantly longer in the RS and LS groups than in the OS group (94.8%, 91.9%, and 86.9%, respectively; p < 0.001). Moreover, the survival benefit of RS was shown in the subgroup analysis of low-risk endometrial cancer patients.
IntroductionEndometrial cancer is the most common malignancy of the female reproductive tract in industrialized countries [1]. Surgery is the first major step in the management of this disease, and the outcomes of surgery can guide the choice of postoperative adjuvant treatment. At present, open surgery (OS), laparoscopic surgery (LS), and robotic surgery (RS) can be used to treat endometrial cancer [2].
Compared to the OS, the LS provides equivalent oncologic outcomes with reduced surgical and postoperative morbidity [3,4]. However, the steep learning curve associated with the LS restricts its widespread application as a surgical treatment for endometrial cancer. The introduction of robot-assisted LS with a relatively shallower learning curve has encouraged more gynecologic oncologists to employ minimally invasive surgery (MIS) over the OS when treating endometrial cancer, and this has resulted in approximately 80% of patients undergoing hysterectomy for cancer by the RS in the United States [5].
Previous research comparing the OS with the LS [6] and the LS with the RS exists [7], along with one meta-analysis comparing all three approaches [8]. However, data are scarce concerning the direct comparison of survival outcomes among the OS, the LS, and the RS for endometrial cancer [9]. Thus, the advantage of the RS relative to the LS and the OS in the treatment of endometrial cancer has not yet been fully determined.
Therefore, in this study, we performed a nationwide population-based cohort study to compare the perioperative and oncologic outcomes of different surgical approaches with the aim of evaluating the advantages of the RS in the staging of endometrial cancer.
Materials and Methods1. Study design and databaseWe performed a nationwide population-based retrospective cohort study by investigating the Korean National Health Insurance Service (NHIS) claims database from January 2012 to December 2016. The Korean NHIS is a mandatory national health insurance program established by the government that covers all forms of health-care utilization, including pharmaceutical services, outpatient and inpatient care, and diagnostic and surgical procedures for the entire population (approximately 51 million people). Hospitals are required to submit all information regarding health-care utilization for reimbursement, and this information is registered in a comprehensive database managed by the NHIS. The NHIS database also contains information on personal demographics and diagnoses identified by the International Classification of Diseases 10th revision (ICD-10) codes.
The Korean government provides additional financial support for patients diagnosed with a “Rare Incurable Disease,” including endometrial cancer, who are registered using a specific code for the “Exempted Calculation of Health Insurance” based on a histopathologic evaluation. Accordingly, detecting patients with endometrial cancer using the cancer-related ICD-10 codes combined with the additional code for the Rare Incurable Disease is considered to be reliable.
Although RS is not covered by the NHIS in South Korea, it has been used in several gynecologic fields as an MIS since 2006 [10]. Since data on the actual cost of RS are not available in the claims database, a direct cost comparison of the total cost according to the mode of surgery was not possible. Instead, a comparison was performed using costs claimed within 30 days of each surgery in the outpatient and emergency department under the diagnosis of endometrial cancer.
2. Study population identificationWomen aged 18 years or older from the database were included in this analysis. Patients who had invalid data on hysterectomy, who had been diagnosed with other types of cancer at the time of hysterectomy, and who had missing sociodemographic information were excluded.
Patients were assigned to each group with the following process. First, among the patients with endometrial cancer, women who had a general anesthesia code and a postoperative pathology examination code were selected. Then, patients who had both hysterectomy procedure and laparoscopy material code were allotted to the LS group. The patients who had only hysterectomy procedure code without a laparoscopy material code were defined as the OS group. Finally, the RS group was defined as the absence of a hysterectomy procedure code and a laparoscopy material code [11].
3. Confounding factorsConfounding factors were extracted from the database, including age group, risk group, modified Charlson comorbidity index (mCCI) score, income level, insurance type, and hysterectomy year. The risk group was determined according to whether the patients were treated with adjuvant therapy, including chemotherapy or radiotherapy, within 6 months after discharge. Comorbidities were assessed using the mCCI, which differed from the standard Charlson comorbidity index by excluding the subject’s age and presence or absence of kidney disease [12]. Comorbidities were considered to be confounding factors when there was a reported correlation between the concomitant chronic disease and the prognosis of cancer [13]. The diagnosis of a comorbidity was defined as a diagnosis at any visit within the one year prior to the hysterectomy date.
4. Statistical analysisTo estimate unbiased causal treatment effects in the national cohort data, we used the inverse probability of treatment weighting (IPTW) method [14]. Demographic and tumor characteristics were compared using chi-squared tests. We used multivariable Cox proportional hazards models to determine the hazard ratio (HR) for predicting recurrence or death after adjustments for confounding variables. Progression-free survival (PFS) was defined as the duration, in days, from the date of surgery to the recurrence or end of follow-up, and overall survival was defined as the interval between the date of surgery and death or end of follow-up, whichever came first. The survival analysis was presented as Kaplan-Meier plots. p-values less than 0.017 (0.05/3=0.017) were considered significant for multiple testing (RS vs. LS, RS vs. OS, LS vs. OS). All statistical analyses were conducted using SAS Enterprise Guide ver. 9.4 (SAS Institute Inc., Cary, NC).
Results1. Study populationAfter the IPTW adjustment, 5,065 patients with endometrial cancer treated with either RS (n=315, 6.2%), LS (n=3,248, 64.1%), or OS (n=1,503, 29.7%) were included in the final analysis (Fig. 1). The three cohorts showed no significant difference in age group, risk group, type of health insurance, income level, mCCI score, comorbidities, and index year (Table 1). RS and LS were more likely than OS to be performed in metropolitan hospitals (p < 0.001).
2. Recovery outcomes and postoperative morbidityPostoperative complications occurred in 21patients (6.8%) in the RS group, 240 (7.4%) in the LS group, and 222 (14.8%) in the OS group, with the overall complication rate being significantly lower in the MIS group (RS and LS) than in the OS group. Disorders of the lymphatic vessels, disruption of operation wounds, and intestinal obstruction were more common in the OS group than in the other two groups (Table 2).
Patients in the MIS (RS and LS) groups showed earlier recovery with respect to the length of hospital stay than those in the OS group (RS vs. LS vs. OS: 10.2 days vs. 10.3 days vs. 13.8, respectively, p < 0.001). There was no difference between the RS and LS groups (Table 2).
3. Cost of outpatient and emergency room visits after surgeryThe number of outpatient visits was significantly lower in the MIS group (RS and LS) compared with the OS group (2.9 vs. 2.9 vs. 3.8 visits: RS vs. LS vs. OS, respectively, p < 0.001). In addition, the total cost of the visits was the lowest in the RS group ($298.00 vs. $348.66 vs. $532.69: RS vs. LS vs. OS, respectively, p < 0.001). The total cost of the emergency room visits after surgery was the lowest in the RS group ($633.14 vs. $781.32 vs. $1,974.44: RS vs. LS vs. OS, respectively, p < 0.001). However, a significant difference was not noted between the RS and LS groups (Table 3).
4. Oncologic outcomesPFS was more favorable in the MIS (RS and LS) group than in the OS group (93.1%, 92.3%, and 87.5%, p < 0.001) (Fig. 2A). The five-year overall survival was significantly longer for the RS and the LS group than the OS group (94.8%, 91.9%, and 86.9%, respectively, p < 0.001) (Fig. 2B). Moreover, according to the univariate and multivariate analyses using the Cox proportional hazards model, the index surgery was a significant factor in predicting overall death, along with prognostic factors such as age group, mCCI score, and risk group (Table 4). The survival benefit of the RS was observed in the subgroup analysis for the low endometrial cancer risk group but not the high-risk group (S1 Table).
DiscussionThis study compared the operative and oncologic outcomes among open, laparoscopic, and robot-assisted staging surgeries in the era of a shift in the standard of care in endometrial cancer management from the OS to MIS [5]. We found that robotic staging surgery did not seem to compromise surgical or survival outcomes when compared to conventional laparoscopic and laparotomy for endometrial cancer. To the best of our knowledge, this population-based cohort study is the largest to compare the oncologic outcomes according to the three modes of surgery in endometrial cancer after the introduction of the RS to the field of gynecologic oncology.
Regarding recovery outcomes, postoperative complications, and cost after the index surgery, the RS was shown to be equivalent to the conventional LS and superior to the OS, which is in accordance with previous studies [15,16]. The reason for this finding may be the RS is gentler, causes minor damage to the internal organs, produces less postoperative pain, and aids faster return to a normal diet and normal activities [17,18].
Any substantial change in the surgical approach to cancer management necessitates evaluation to ensure survival is not compromised. In terms of the survival outcomes of the MIS for endometrial cancer, most of the available studies compared the OS with the LS rather than with the RS and indicated that laparoscopy was a favorable alternative for patients with endometrial cancer [3,19]. In the present study, we compared the survival outcomes between IPTW propensity score-matched groups of patients undergoing RS, LS, and OS after the introduction of robot-assisted LS. Our results showed that the adoption of the RS did not compromise the survival outcomes compared with other modes of surgery.
Several studies have reported comparative long-term oncological outcomes between the OS and the RS for endometrial cancer. Corrado et al. [20] observed that the 3-year overall survival was 86.7% and 91.5% and the 3-year PFS was 92.1% and 91.5% for OS and RS, respectively. Likewise, Cardenas-Goicoechea et al. showed that there were no significant differences in survival between robotic and laparoscopic surgeries (3-year PFS was 88.4% and 83.3% and 3-year overall survival was 93.6% and 93.3% with LS and RS, respectively) [21]. Moreover, Brudie et al. [22] reported a 3-year PFS of 89.3% and 3-year overall survival of 89.1%, and Kilgore et al. [23] noted a 5-year overall survival of 89.1% in patients who underwent RS for endometrial cancer. In the present analysis, we demonstrated comparable survival outcomes (5-year PFS, 93.1%, and 5-year overall survival, 94.8%) in the RS group.
Two randomized controlled trials (RCTs) and a large-scale meta-analysis have reported similar overall survival for the MIS and the OS, and that MIS is correlated with reduced surgical complications in non-metastatic endometrial cancer [3,19,24]. Though RCTs are accepted as having excellent internal validity, they do not necessarily describe the influence of a specific treatment in an entire population since frail subgroups tend to be excluded owing to strict inclusion criteria for enrollment [25]. Our population-based study included the entire population of Korea who were treated under the diagnosis of endometrial cancer and a subgroup analysis was performed. Notably, our subgroup analysis indicated that the positive correlation between RS and favorable survival outcomes was present among low-risk patients, and similar survival was observed among high-risk women. This finding showed that the RS for endometrial cancer is, therefore, not only considered oncologically safe but is also likely to provide clear benefits for low-risk endometrial cancer patients in general.
Our results on the survival outcomes are consistent with those of the previously reported nationwide retrospective cohort study, which analyzed a population-based registry. Patients with early-stage endometrial cancer derived from the National Cancer Database of the United States were analyzed [26]. The overall survival after RS improved significantly compared with OS when adjustments were made for age, surgical year, comorbidity, race, lymph-node yield, stage, adjuvant treatment, and economic status [26]. However, this analysis did not include patients treated with LS in the comparison. Another analysis of elderly middle-class patients with early-stage endometrial cancer from the registry of Medicare, a U.S. national insurance program, was performed, and comparable overall survival rates were observed for the MIS and the OS [27]. Unlike our study, this study did not distinguish between the RS and the LS, and the overall MIS was compared with the OS. The present study, encompassing 5,065 Korean patients with endometrial cancer, included all ages and socioeconomic statuses and found that the RS and the LS were associated with improved survival compared with the OS.
We believe that our data are reliable for comparing the operative and oncologic outcomes in the real world because the cancer diagnostic codes and demographic variables registered in the Korean NHIS database are deemed accurate. Nevertheless, we should interpret the findings of our study in the context of the limitations associated with this nationwide retrospective study. For example, variables such as surgical stage or cell types, which may influence the oncologic outcomes of endometrial cancer, were not considered. The retrospective nature of our study and unmeasured confounding variables are major limitations. Also, the number of patients who underwent the RS was relatively small compared to the LS or the OS. Further, potential selection bias, especially owing to the selection of patients who can undergo RS, may also exist.
In conclusion, our nationwide cohort study provides further evidence for the RS being a safe surgical alternative to the LS and the OS, especially in low-risk endometrial cancer patients, offering surgical and oncologic outcomes equivalent to other surgical approaches.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine (Seoul, Korea) (IRB No. 4-2019-0817). Informed consent requirements were waived because the study was based on routinely collected administrative data, and patient data were kept anonymous. Author Contributions Conceived and designed the analysis: Eoh KJ, Nam EJ, Kim SW, Kim YT. Collected the data: Shin M, Kim SJH, Kim JA. Contributed data or analysis tools: Eoh KJ, Nam EJ, Kim SW, Shin M, Kim SJH, Kim JA, Kim YT. Performed the analysis: Eoh KJ, Shin M, Kim SJH, Kim JA, Kim YT. Wrote the paper: Eoh KJ, Kim YT. AcknowledgmentsThe authors would like to thank the Korean National Health Insurance Service for their cooperation.
Fig. 1Flow diagram of the study population. IPTW, inverse probability of treatment weighting; mCCI, modified Charlson comorbidity index. 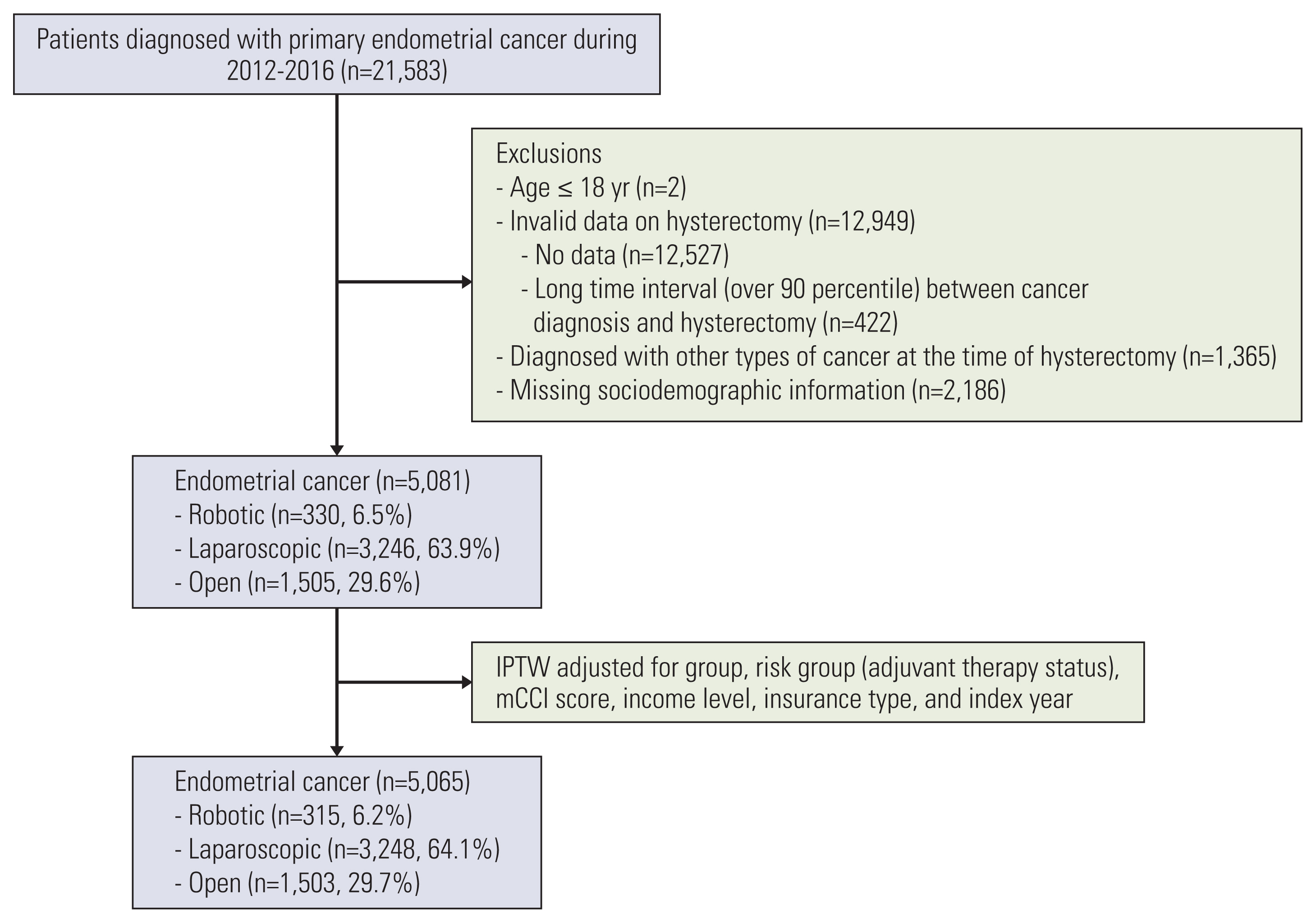
Fig. 2IPTW-adjusted progression-free survival (A) and overall survival (B) for patients with endometrial cancer. IPTW, inverse probability of treatment weighting. 
Table 1Baseline characteristics for patients with endometrial cancer Table 2Hospital stay and postoperativecomplications Table 3Cost of endometrial cancer-related outpatient and ER visits after operation Table 4Univariate and multivariate hazard ratio for overall death References2. Wright JD, Barrena Medel NI, Sehouli J, Fujiwara K, Herzog TJ. Contemporary management of endometrial cancer. Lancet. 2012;379:1352–60.
3. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J Clin Oncol. 2012;30:695–700.
4. Walker JL, Piedmonte MR, Spirtos NM, Eisenkop SM, Schlaerth JB, Mannel RS, et al. Laparoscopy compared with laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group Study LAP2. J Clin Oncol. 2009;27:5331–6.
5. Lau S, Vaknin Z, Ramana-Kumar AV, Halliday D, Franco EL, Gotlieb WH. Outcomes and cost comparisons after introducing a robotics program for endometrial cancer surgery. Obstet Gynecol. 2012;119:717–24.
6. Chu LH, Chang WC, Sheu BC. Comparison of the laparoscopic versus conventional open method for surgical staging of endometrial carcinoma. Taiwan J Obstet Gynecol. 2016;55:188–92.
7. Vuorinen RK, Maenpaa MM, Nieminen K, Tomas EI, Luukkaala TH, Auvinen A, et al. Costs of robotic-assisted versus traditional laparoscopy in endometrial cancer. Int J Gynecol Cancer. 2017;27:1788–93.
8. Ran L, Jin J, Xu Y, Bu Y, Song F. Comparison of robotic surgery with laparoscopy and laparotomy for treatment of endometrial cancer: a meta-analysis. PLoS One. 2014;9:e108361.
9. Jorgensen SL, Mogensen O, Wu CS, Korsholm M, Lund K, Jensen PT. Survival after a nationwide introduction of robotic surgery in women with early-stage endometrial cancer: a population-based prospective cohort study. Eur J Cancer. 2019;109:1–11.
10. Kim YT, Kim SW, Hyung WJ, Lee SJ, Nam EJ, Lee WJ. Robotic radical hysterectomy with pelvic lymphadenectomy for cervical carcinoma: a pilot study. Gynecol Oncol. 2008;108:312–6.
11. Kang HW, Yun SJ, Chung JI, Choi H, Kim JH, Yu HS, et al. National practice patterns and direct medical costs for prostate cancer in Korea across a 10 year period: a nationwide population-based study using a national health insurance database. BMC Health Serv Res. 2019;19:408.
12. Mehrotra R, Kermah D, Fried L, Kalantar-Zadeh K, Khawar O, Norris K, et al. Chronic peritoneal dialysis in the United States: declining utilization despite improving outcomes. J Am Soc Nephrol. 2007;18:2781–8.
13. Read WL, Tierney RM, Page NC, Costas I, Govindan R, Spitznagel EL, et al. Differential prognostic impact of comorbidity. J Clin Oncol. 2004;22:3099–103.
14. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79.
15. Salehi S, Avall-Lundqvist E, Legerstam B, Carlson JW, Falconer H. Robot-assisted laparoscopy versus laparotomy for infrarenal paraaortic lymphadenectomy in women with high-risk endometrial cancer: a randomised controlled trial. Eur J Cancer. 2017;79:81–9.
16. Bergstrom J, Aloisi A, Armbruster S, Yen TT, Casarin J, Leitao M Jr, et al. Minimally invasive hysterectomy surgery rates for endometrial cancer performed at National Comprehensive Cancer Network (NCCN) Centers. Gynecol Oncol. 2018;148:480–4.
17. Jorgensen SL, Mogensen O, Wu C, Lund K, Iachina M, Korsholm M, et al. Nationwide introduction of minimally invasive robotic surgery for early-stage endometrial cancer and its association with severe complications. JAMA Surg. 2019;154:530–8.
18. Galaal K, Donkers H, Bryant A, Lopes AD. Laparoscopy versus laparotomy for the management of early stage endometrial cancer. Cochrane Database Syst Rev. 2018;10:CD006655.
19. Siesto G, Uccella S, Ghezzi F, Cromi A, Zefiro F, Serati M, et al. Surgical and survival outcomes in older women with endometrial cancer treated by laparoscopy. Menopause. 2010;17:539–44.
20. Corrado G, Cutillo G, Pomati G, Mancini E, Sperduti I, Patrizi L, et al. Surgical and oncological outcome of robotic surgery compared to laparoscopic and abdominal surgery in the management of endometrial cancer. Eur J Surg Oncol. 2015;41:1074–81.
21. Cardenas-Goicoechea J, Shepherd A, Momeni M, Mandeli J, Chuang L, Gretz H, et al. Survival analysis of robotic versus traditional laparoscopic surgical staging for endometrial cancer. Am J Obstet Gynecol. 2014;210:160.
22. Brudie LA, Backes FJ, Ahmad S, Zhu X, Finkler NJ, Bigsby GE 4th, et al. Analysis of disease recurrence and survival for women with uterine malignancies undergoing robotic surgery. Gynecol Oncol. 2013;128:309–15.
23. Kilgore JE, Jackson AL, Ko EM, Soper JT, Van Le L, Gehrig PA, et al. Recurrence-free and 5-year survival following robotic-assisted surgical staging for endometrial carcinoma. Gynecol Oncol. 2013;129:49–53.
24. Janda M, Gebski V, Davies LC, Forder P, Brand A, Hogg R, et al. Effect of total laparoscopic hysterectomy vs total abdominal hysterectomy on disease-free survival among women with stage I endometrial cancer: a randomized clinical trial. JAMA. 2017;317:1224–33.
25. Booth CM, Tannock IF. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer. 2014;110:551–5.
|
|
||||||||||||||||||||||||||||||||||||||||||||||