AbstractPurposeWe developed a new method of detecting circulating tumor cells (CTCs) in liver cancer patients by constructing cell blocks from peripheral blood cells, including CTCs, followed by multiple immunohistochemical analysis.
Materials and MethodsCell blockswere constructed from the nucleated cell pellets of peripheral blood afterremoval of red blood cells. The blood cell blocks were obtained from 29 patients with liver cancer, and from healthy donor blood spikedwith seven cell lines. The cell blocks and corresponding tumor tissues were immunostained with antibodies to seven markers: cytokeratin (CK), epithelial cell adhesion molecule (EpCAM), epithelial membrane antigen (EMA), CK18, α-fetoprotein (AFP), Glypican 3, and HepPar1.
ResultsThe average recovery rate of spiked SW620 cells from blood cell blocks was 91%. CTCs were detected in 14 out of 29 patients (48.3%); 11/23 hepatocellular carcinomas (HCC), 1/2 cholangiocarcinomas (CC), 1/1 combined HCC-CC, and 1/3 metastatic cancers. CTCs from 14 patients were positive for EpCAM (57.1%), EMA (42.9%), AFP (21.4%), CK18 (14.3%), Gypican3 and CK (7.1%, each), and HepPar1 (0%). Patients with HCC expressed EpCAM, EMA, CK18, and AFP in tissue and/or CTCs, whereas CK, HepPar1, and Glypican3 were expressed only in tissue. Only EMA was significantly associated with the expressions in CTC and tissue. CTC detection was associated with higher T stage and portal vein invasion in HCC patients.
IntroductionHepatocellular carcinoma (HCC) is a leading cause of cancer-related deaths worldwide. The highest liver cancer rates are found in East and South-East Asia. HCC is the major histological subtype among primary liver cancers, accounting for 70% to 85% of the liver cancer burden worldwide [1]. Most HCC patients present with intermediate or advanced-stage disease, for which both curative and palliative treatments are unsatisfactory [2]. Poor outcomes are generally associated with underlying cirrhosis, a high recurrence rate and late diagnosis. The high recurrence rate and aggressiveness are related to tumor characteristics, including early vascular invasion. Vascular invasion is an important prognostic factor in patients with HCC, along with patient age, size and number of tumors, presence of a tumor capsule, histological grade, and pathological TNM stage [3]. Vascular invasion affects postoperative survival and recurrence [3,4].
Circulating tumor cells (CTCs), defined as tumor cells circulating in the peripheral blood of cancer patients, are shed by either the primary tumor or a metastatic site into the circulation via vascular invasion or intravasation [5]. CTCs are a potential source of biological information that can be used to predict clinical outcome. The isolation and characterization of CTCs is difficult because these cells are extremely rare [5,6]. Over the past decade, many methods have been developed for the detection of CTCs in the peripheral blood. Despite the variety of these methods, final confirmation of CTCs depends on their expression of epithelial cell adhesion molecule (EpCAM). EpCAM is expressed ubiquitously, albeit at variable levels, in epithelial cells and their corresponding cancers, but is not expressed in blood cells [7,8]. However, because EpCAM expression on cell surfaces is down-regulated in some types of tumor cells, methods that use the anti-EpCAM affinity molecule to detect CTCs will not identify CTCs negative for EpCAM expression. This can be a crucial limitation in CTC analysis, as the cancer cells are heterogeneous [6]. EpCAM-based detection of CTCs has been approved to predict prognosis in patients with breast, colorectal, and prostate cancers. However, identification and predictive value of CTCs in HCC is still under investigation [9,10]. EpCAM is expressed by embryonic liver and hepatic stem cells, but only by a subset of HCCs, making EpCAM-based CTC studies in patients with HCC problematic. Park et al. [11] reported that EpCAM (clone VU-1D9) expressed in 48.7% of the surgically resected HCC specimens.
We recently developed a new method of detecting CTCs by constructing cell blocks from peripheral blood cells including CTCs, followed by multiple immunohistochemical analysis. This study was designed (1) to compare immunohistochemical expression of multiple tumor markers in peripheral blood-derived CTCs and liver cancer tissue; (2) to comprehensively investigate the CTCs in these patients; (3) to evaluate the relationship between clinicopathological features and the expression of markers by CTCs; and (4) to investigate the clinicopathological utility of cell block method in the detection of CTCs from liver cancer patients.
Materials and Methods1. Cell lines and cultureTo identify candidate CTC markers for HCC cells, six human HCC cell lines were selected, along with two colon cancer cell lines (SW480 and SW620) as controls for epithelial-mesenchymal transition (EMT) phenotype. SW480 and SW620 cells are isogenic and derived from primary carcinoma and lymph node metastasis respectively of one patient [12].
The human HCC cell lines HepG2, PLC/PRF5, and Huh-7 were maintained as monolayer cultures in Dulbecco’s modified Eagle medium, supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin, and 100 μg/mL streptomycin (Gibco, Grand Island, NY) in a humidified 5% CO2/95% air atmosphere at 37°C. The human HCC cell lines SNU-182, SNU-387, and SNU-449 were cultured in RPMI-1640 supplemented with 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin. The colon cancer cell lines SW480 and SW620 were grown in Leibovitz's L-15 Medium containing 10% FBS, 100 units/mL penicillin, and 100 μg/mL streptomycin.
2. PatientsPeripheral blood samples were collected from 23 patients with HCC, two with cholangiocarcinoma (CC), one with combined HCC-CC, and three with metastatic carcinomas (two colorectal adenocarcinomas and one nasopharyngeal carcinoma) who were diagnosed between August 2013 and April 2014 at the National Cancer Center (NCC), Korea. Each tumor was classified according to World Health Organization classification criteria [13] and the American Joint Committee on Cancer (AJCC) staging system [14]. Blood samples were collected at initial diagnosis before the initiation of therapy in all patients, except one whose HCC recurred after surgical resection. From the latter patient, a blood sample was drawn during chemotherapy due to tumor progression. Tumor tissues were taken by needle biopsy in 27 patients, and by surgical resection in two patients. Transarterial chemoembolization was performed in seven patients, and chemotherapy was given in 16 patients. The study protocol (NCCNCS 12660) was approved by the Institutional Review Board of the NCC, and this study was conducted in accordance with the Helsinki Declaration. All study participants provided their written informed consent for the study.
3. Western blottingCells were lysed with lysis buffer (5 mM/L ethylenediaminetetraacetic acid [EDTA]; 300 mM/L NaCl; 0.1% NP-40; 0.5 mM/L NaF; 0.5 mM/L Na3VO4; 0.5 mM/L phenylmethylsulfonyl fluoride; and 10 μg/mL each of aprotinin, pepstatin, and leupeptin; Sigma, St. Louis, MO) and centrifuged at 15,000 ×g for 30 minutes. The concentrations of supernatant proteins were analyzed by Bradford reagent (Bio-Rad, Hercules, CA). Samples containing 50 μg total protein were electrophoresed in 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA), which were blocked by incubation for 2 hours at 4°C in 1% Tween 20-TBS buffer containing 1.5% non-fat dry milk (Bio-Rad) and 1 mM MgCl2. The membranes were incubated for 2 hours at room temperature with antibodies against E-cadherin (R&D Systems, Minneapolis, MN), vimentin (Santa Cruz Biotechnology, Dallas, TX), SLUG (Santa Cruz Biotechnology), SNAIL (Santa Cruz Biotechnology), and β-actin (Santa Cruz Biotechnology), washed three times for 15 minutes each with blocking solution and incubated with diluted horseradish peroxidase–conjugated secondary antibodies (Southern Biotech, Birmingham, AL) for 1 hour room temperature. The membranes were again washed three times for 15 minutes each with blocking solution, incubated with WEST-ZOL plus chemiluminescence reagent (iNtRON Biotechnology, Seoul, Korea) for 1 minute, and exposed to film (Kodak Blue XB-1).
4. Cell block constructionBlood samples (5 mL) were collected in buffered EDTA, diluted with an equal volume of phosphate buffered saline (PBS) solution (Welgene, Daegu, Korea), and layered onto Ficoll-Hypaque (Ficoll-Paque, GE Healthcare Bio-Sciences, Pittsburgh, PA). The samples were centrifuged in 15 mL disposable centrifuge tubes at 2,000 rpm for 20 minutes. After drawing off the upper layer, the lymphocyte layer was transferred to a 50 mL centrifuge tube, to which was added 3 volumes of PBS followed by centrifugation at 1,500 rpm for 10 minutes. After removing the supernatant, the cell pellet was mixed with an equal volume of 30 mg/mL gelatin (Sigma-Aldrich, Seoul, Korea) and the mixture (gel block) was refrigerated for 20 minutes at 4ºC and fixed with neutralized buffered formalin overnight. The fixed cell pellet mixture was wrapped in crayon paper, placed in a cassette, and stored in 80% ethanol. The pellets were next processed in the automatic tissue processor using a 13-hour schedule, consisting of 80% ethanol with 1 change (2.5 hours), 95% ethanol (1 hour), 100% ethanol, with four changes (1 hour each), 1:1 ethanol/xylene (1 hour); xylene with three changes (1 hour each), paraffin wax at 60°C (1 hour); and paraffin wax at 60°C with vacuum impregnation at 20 lb (30 minutes).
5. Cell spiking testThe detection sensitivity of the cell block method was evaluated by a cell spiking test. SW620 cells were mixed with 5 mL aliquots of peripheral blood obtained from a healthy volunteer at ratios of 1:104, 1:105, and 1:106 white blood cells (equivalent to 4,000, 400, 40 spiked SW620 cells). Cell blocks were constructed as described and cut serially. The sections were incubated with antibody to cytokeratin (CK) and the numbers of CK-positive cells in sections were counted. To avoid overlapped counting of CTCs, cell blocks were serially cut at a thickness of 4 μm, and alternate sections were used for immunohistochemistry, with intervention sections discarded based on the assumption that the mean diameter of CTCs was greater than 8 μm [15]. The CK-positive cells were counted in the entire blocks. The tests were repeated at least three times, and the mean recovery rate was calculated by the ratio of CK-positive cell number to spiked cell number in the tests of each dilution concentration.
6. ImmunohistochemistryUsing the BenchMark XT Slide Preparation System (Ventana Medical Systems Inc., Tucson, AZ), 4 μm sections from peripheral blood cell blocks and corresponding tumor paraffin blocks (liver biopsy or resected specimens) were immunostained with antibodies to CK (clone AE1/AE3, 1:100, Dako Cytomation, Glostrup, Denmark), EpCAM (clone VU-1D9, 1:2,000, Calbiochem, San Diego, CA), epithelial membrane antigen (EMA; clone GP1.4, 1:200, Novocastra, Leica Biosystems, Heidelberg, Germany), CK18 (clone DC10, 1:200, Dako Cytomation), HepPar1 (clone OCH1E5, 1:800, Dako Cytomation), Glypican3 (clone 1G12, 1:200, BioMosaics, Burlington, VT) and α-fetoprotein (AFP; polyclonal, 1:800, Dako Cytomation). At least two sections were stained with each antibody, with positivity defined as the unequivocal expression of any marker in the cytoplasm and/or membrane. All slides were reviewed by two pathologists (H.J.C. and S.J.N.). Criteria for CTCs included cell size (1.5 times larger than white blood cells), large nuclei with high nuclear/cytoplasmic ratio, irregularity of the nuclear membrane, presence of cytoplasm (not bare nuclei), and microemboli formation. Cells that were non-neoplastic, including bare nuclei of megakaryocytes, EMA (+)-hematopoietic cells including immature precursors and plasma cells, and CK (+) contaminated keratinocytes, were excluded. The presence of atypical cells expressing any marker was regarded as positive for CTC.
Results1. Protein expression of human cancer cell linesTo detect CTC markers in liver cancer, the expression of epithelial and mesenchymal markers (E-cadherin, vimentin, SLUG, and SNAIL) was analyzed in human cancer cell lines (Fig. 1). HepG2, Huh7, and SNU-182 were positive for E-cadherin by western blot analysis but negative for vimentin and SNAIL, indicating that they were epithelial cell lineages. SNU-387 and SNU-449 cells were positive for vimentin and SNAIL but negative for E-cadherin, indicating a mesenchymal character. PLC/RPF5 cells expressed both epithelial and mesenchymal markers.
2. Cell spiking testCell spiking tests were performed to determine the detection efficiency of the cell block method. Each 5 mL blood sample yielded cell blocks containing an average of 33 sections, of 4 μm thickness each (excluding the discarded sections). The mean recovery rate of spiked SW620 cells from blood cell blocks was 91.2±22.8%. In spiking of SW620 cells in normal blood samples at a ratio of 1:104 white blood cells, the mean number of CTCs per section was 113.4±53.0; at 1:105, 11.1±18.1; and at 1:106, 1.1±1.5.
3. Immunohistochemical expression of candidate markers in peripheral blood cell blocks containing HCC cell linesTo identify useful markers, healthy donor blood was spiked with the human HCC cell lines HepG2, PLC/PRF5, SNU-182, SNU-387, SNU-449, and Huh7 at a ratio of 1:104. Cell blocks were constructed and sections were immunostained with antibodies to CK, EpCAM, EMA, CK18, AFP, Glypican3, and HepPar1 (Fig. 2). All six HCC cell lines expressed CK18. Glypican3 and AFP were expressed by the three epithelial HCC cell lines, SNU-182, Huh7, and HepG2, and by the semi-epithelial cell line PLC/RPF5. The three epithelial cell lines also expressed CK and EpCAM, but they did not express EMA or HepPar1. The SNU-387 cell line, which showed mesenchymal features in western blot analysis, expressed both EpCAM and EMA. The semi-mesenchymal cell line, PLC/RPF5, expressed only HepPar1 (Table 1).
4. Detection of CTCs from liver cancer patients and association with clinicopathologic features
Table 2 summarizes the clinical characteristics of the 29 liver cancer patients. Their median age was 59 years (range, 38 to 80 years) and 89.7% were male. Of the 29 patients, 23 had HCC, two had CC, one had combined HCC-CC and three had metastatic carcinomas. Most patients were stage T3b (55.2%) and stage IIIB (41.4%) based on the AJCC/Union for International Cancer Control TNM staging system. CTCs were positive in 14 patients (48.3%) including 11 patients with HCC, one CC, one HCC-CC, and one metastatic nasopharyngeal carcinoma.
CTC positivity was associated with higher T stage in liver cancer patients (p=0.002), and in patients with HCC (p=0.010). CTCs were more frequently found in HCC patients with than without portal vein invasion (p=0.036). There were no significant associations between the presence of CTC and histologic type, size, stage, multiplicity, or serum AFP concentration. Of the 14 patients positive for CTCs, eight (57.1%) were positive for EpCAM, six (42.9%) for EMA, three (21.4%) for AFP, two (14.3%) for CK18, one each (7.1%) for Glypican3 and CK, and none (0%) for HepPar1 (Table 3).
5. Comparison of immunohistochemical expression profiles of CTCs and their primary liver cancersFormalin-fixed paraffin-embedded tissue blocks of liver cancer biopsy specimens were retrieved for 28 liver cancer patients. To compare marker expression in CTCs and tumor tissue, representative sections of resected liver cancer tissues were immunohistochemically stained with antibodies to CK, EpCAM, EMA, CK18, AFP, Glypican3, and HepPar1 (Figs. 3 and 4). A comparison of marker expression profiles in CTC and tissue samples found that EMA was the only marker associated with HCC (p=0.039) (Table 4). HCC patients positive for CTC were positive for the expression of EpCAM, EMA, CK18, and AFP in tissue and/or CTC, whereas CK, HepPar1, and Glypican3 were mostly expressed in tissue only. In particular, CTCs were positive for EpCAM in three of 13 patients (23.1%) with tumor tissues negative for EpCAM, and CTCs were positive for EMA in one of 14 patients (7.1%) with tumor tissues negative for EMA.
6. Prognostic analysis by CTCOverall survival (OS) tended to be poorer in liver cancer or HCC patients with than without CTCs, although the associations were not statistically significant (Fig. 5A and E). OS tended to be also poorer in patients of liver cancer (p=0.056) or HCC (p=0.058) with CTCs positive than negative for AFP (Fig. 5D and H). OS tended to be poorer in patients with CTCs positive than negative for EpCAM, but the differences were not statistically significant (Fig. 5B and F). EMA expression by CTCs had no prognostic significance (Fig. 5C and G).
DiscussionCTCs may have potential as liquid biopsy biomarkers. However, CTCs are rare, comprising as few as one per 106-109 hematologic cells in the blood of patients with metastatic cancer, making their purification technically challenging [9,16,17]. Methods of CTC enrichment include immune-affinity, physical properties, direct analysis, and mutation detection/genomic analysis [5], but none has adequate sensitivity and specificity. Nonspecific enrichment techniques include those based on physicochemical properties (e.g., size and density), including density gradient centrifugation, lysis buffers, cytocentrifugation, and isolation by size of epithelial tumor cells (ISET method) [18]. Specific enrichment techniques use methods based on markers expressed by CTCs, including magnetic separation and epithelial immunoSPOT.
Several techniques are used to detect CTCs in HCC patients. For example, a magnetic bead method isolated HCC cells based on the expression of a specific hepatocyte marker, biotinylated asialofetuin (a ligand of asialoglycoprotein receptor), with subsequent identification by immunofluorescence staining with antibody to HepPar 1 [19]. The average recovery rate at each spiking level was 61% or higher, and CTCs were identified in 69 of the 85 HCC patients (81%). However, the hepatocyte marker was also expressed on the circulating non-neoplastic hepatocytes, and the marker might be unsuitable for undifferentiated cancer cells. Circulating CD45– CD90+ CD44+ cancer stem cells (CSCs) detected by multicolor flow cytometry predict post-hepatectomy HCC recurrence with high accuracy [20]. In that study, 92% of HCC patients with very limited disease were positive for circulating CSC, suggesting systemic disease in almost all patients, perhaps limiting the specificity of the method.
This study validated the utility of the cell block method for detecting CTCs, demonstrating the specificity of this technique compared with other methods. Although techniques for enrichment and detection of CTC have been developed, final confirmation of CTC depends on immunofluorescence [5,21]. Immunofluorescent stain is limited in the cytologic evaluation of cellular details, and yields false-positive (non-tumor cell) or false-negative (non-reactive tumor cell) results. Our cell block method could overcome this limitation by immunohistochemical stain with cytomorphologic evaluation. Immunohistochemical analysis of cell block is a traditional cytologic diagnostic method, widely used in pathologic laboratories. However, it had not been applied to examine CTC, since blood cells without extracellular matrix cannot be aggregated to construct cell blocks. We developed a new method to construct cell blocks from nucleated blood cells, and we applied multiple immunohistochemical analysis using cell blocks. To identify useful immunohistochemical markers for CTC in liver cancer patients, cell blocks from HCC cell lines and blood samples from liver cancer patients were immunostained with antibodies to CK, EpCAM, EMA, CK18, AFP, Glypican3, and HepPar1. Our results showed that EpCAM and EMA were the most practical of the seven tumor markers. CTCs tended to be associated with higher T stage and portal vein invasion. Moreover, patients positive for CTCs tended to have poorer survival, although the differences were not statistically significant. OS (p=0.056) tended to be poorer in liver cancer patients with CTCs positive than negative for AFP.
The CellSearch system (Veridex, Raritan, NJ) is a semiautomated device that detects and counts EpCAM-positive/cytokeratin-positive CTCs, defined as oval or round cells, containing a morphologically intact nucleus (DAPI staining), with positive expression of cytokeratin and negative expression of CD45 [22]. Using these criteria, 18 of 59 HCC patients and one of 19 patients with cirrhosis or benign hepatic tumor were positive for CTC. A combination of the CellSearch system and quantitative real-time reverse-transcriptase polymerase chain reaction assays showed presence of CTCs in 66.67% of patients and that stem cell-like markers (CD133 and ABCG2) are co-expressed in EpCAM-positive CTCs [23].
Although EpCAM is highly expressed in many human cancers of epithelial cell origin, it is expressed in only 35% of patients with HCC [24,25]. In the adult liver, hepatocytes are negative and bile duct epithelium is positive for EpCAM. Therefore, comparisons between CTC and primary HCC tissue are necessary for evaluating EpCAM expression on CTC, but results of the comparison between CTC and primary tumor tissue are lacking. EpCAM-positive tumor cells in HCC have been associated with enhanced tumor growth and invasiveness and shorter survival. HCC cells expressing EpCAM appear to be stem cell-like and associated with a more aggressive phenotype [26,27], suggesting that they may have the potential to initiate metastases [8,22]. However, EpCAM expression is down-regulated during the EMT, a transition associated with stem cell features. EMT is a key developmental program that is often activated during cancer invasion and metastasis, with several studies showing a direct link between EMT and a gain of epithelial stem cell properties [28,29].
We found that 23.1% of EpCAM-negative patients had CTCs positive for the expression of EpCAM, and 7.1% of those with EMA-negative HCCs had CTCs positive for EMA. When marker expression profiles were compared in CTC and tissue samples, EMA was the only marker associated with HCC. We also observed that HCC patients positive for CTCs had tumors and/or CTCs positive for the expression of EpCAM, EMA, CK18, and AFP, whereas tumors, but not CTCs, were positive for pan-CK, HepPar1, and Glypican3. Although CTCs showed various EpCAM expression patterns, with EpCAM restricted to detecting CTCs, the near completion of CTC detection requires the use of multiple markers, including EpCAM, EMA, and AFP. The discrepancy in tissue marker and CTC marker in HCC patients may be due to loss of tissue-specific differentiation in CTC, resulting from EMT and gain of stem cell properties [26-29].
The major limitation of this study was the limited number of patients. In addition, non-neoplastic control tissue was not included. Nevertheless, our results suggest that the cell block method is a clinically valid and specific technique for detecting CTCs compared with other methods, in terms of immunohistochemical comparison of primary tumor tissue and peripheral blood cell block. Although this method may be limited in detection of very rare CTCs and quantification of CTC numbers in blood, it has several merits: it does not need any special apparatus, is economic, and allows multiple examinations. Our results could provide a framework for further stussdies and a basis for future application of routine cytologic diagnosis for CTCs.
AcknowledgmentsThis study was supported by the Converging Research Center Program funded by the Ministry of Science, ICT and Future Planning, Republic of Korea (Project No. 2013K000271) and National Cancer Center, Korea (grant No. 1510520). The authors thank Ms. Jin Sook Kim and Ms. Sook-Kyung Lee at the Liver and Pancreatobiliary Cancer Branch, National Cancer Center, Korea for their help with administrative support and collection of blood samples.
Fig. 1.Western blot analysis of expression of E-cadherin, vimentin, SLUG, and SNAIL proteins in human liver cancer cell lines. β-Actin was used as a loading control. HepG2, Huh7, and SNU-182 showed epithelial characteristics. SNAIL, SNU-387, and SNU-449 showed mesenchymal characteristics. PLC/RPF5 showed semi-epithelial characteristics. 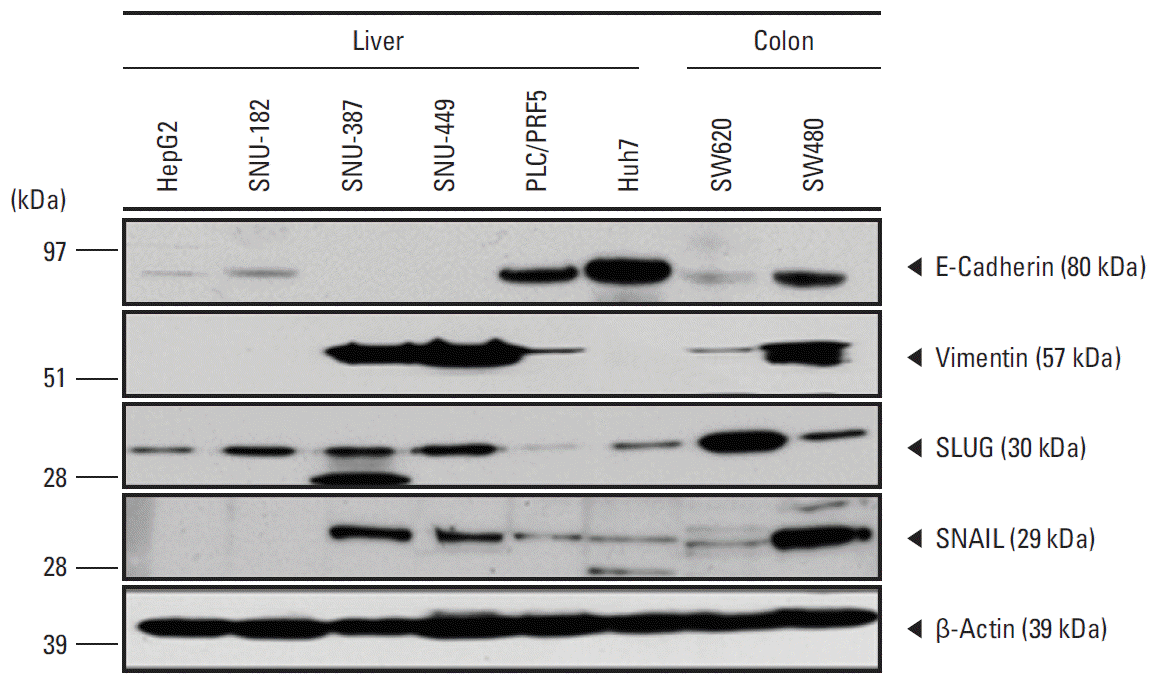
Fig. 2.Histologic and immunohistochemical images (×400) of peripheral blood cell block containing spiked hepatocellular carcinoma cell line HepG2. The HepG2 cells show strong expression of cytokeratin (CK), Glypican3, and α-fetoprotein (AFP), and weak expression of epithelial cell adhesion molecule (EpCAM), but the cells are negative for HepPar1. 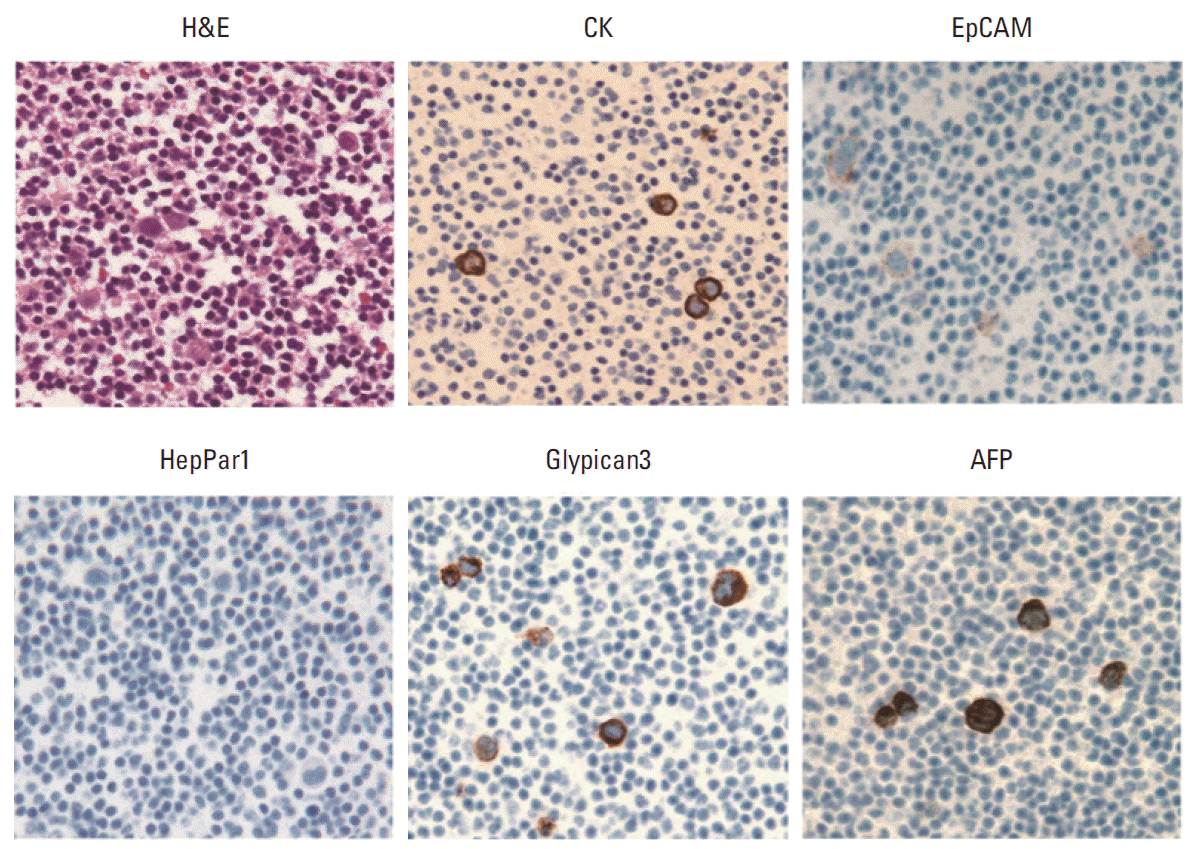
Fig. 3.Representative images (×400) of marker expression in circulating tumor cell (CTC) and hepatocellular carcinoma (HCC) tissue samples from three patients. All three CTCs showed strong cytoplasmic expression of epithelial cell adhesion molecule (EpCAM), but their corresponding HCC tissues were negative for EpCAM. The pairs of samples showed identical expression patterns for cytokeratin 18 (CK18), α-fetoprotein (AFP), epithelial membrane antigen (EMA), and Glypican3. 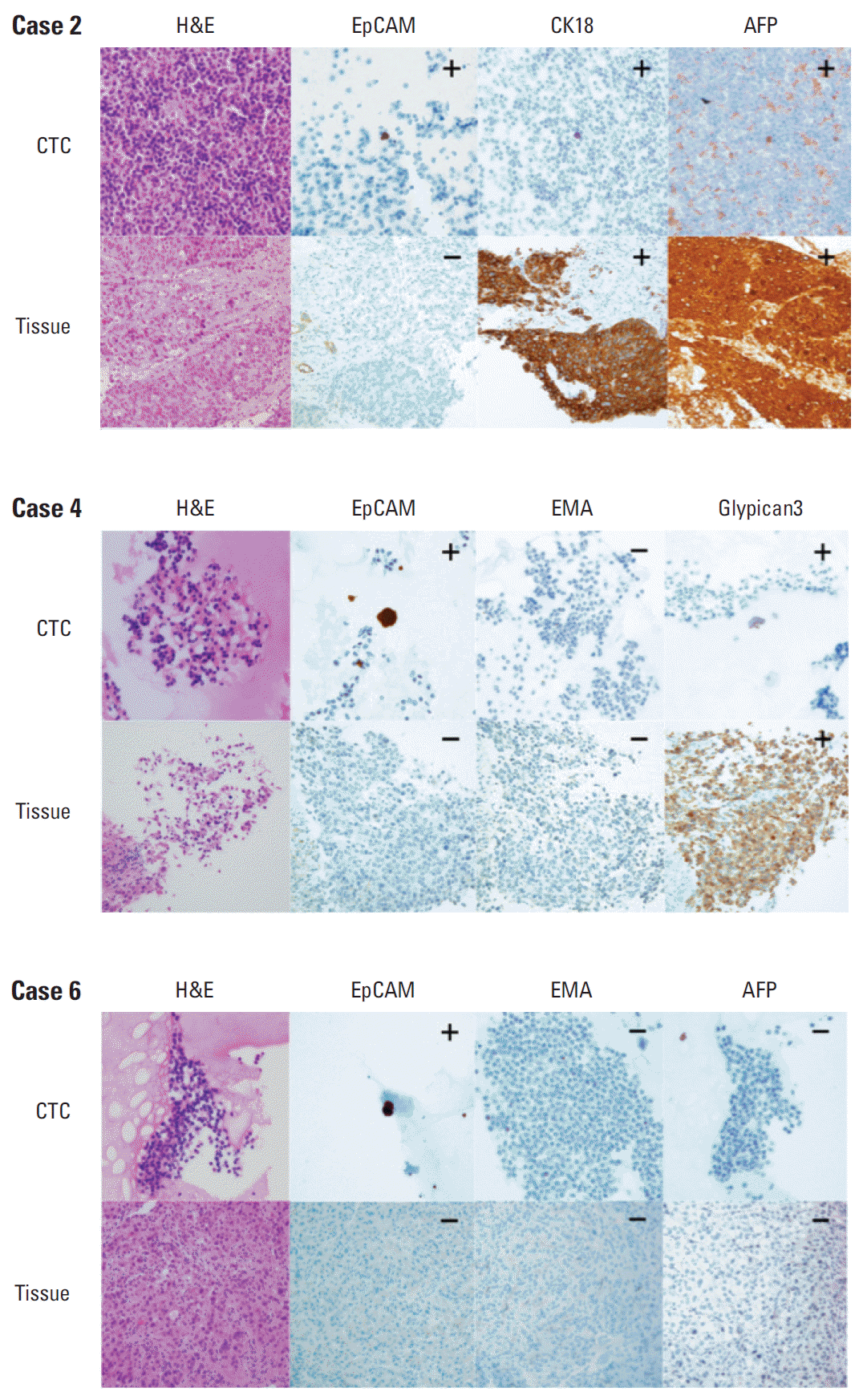
Fig. 4.Association of tumor marker expression in circulating tumor cells (CTCs) and tumor tissue of the 14 hepatocellular carcinoma (HCC) patients positive for CTCs. Three patients with HCC showed aberrant epithelial cell adhesion molecule (EpCAM) expression in CTC. Two patients with HCC showed aberrant epithelial membrane antigen (EMA) expression in CTC. AFP, α-fetoprotein; CK, cytokeratin. 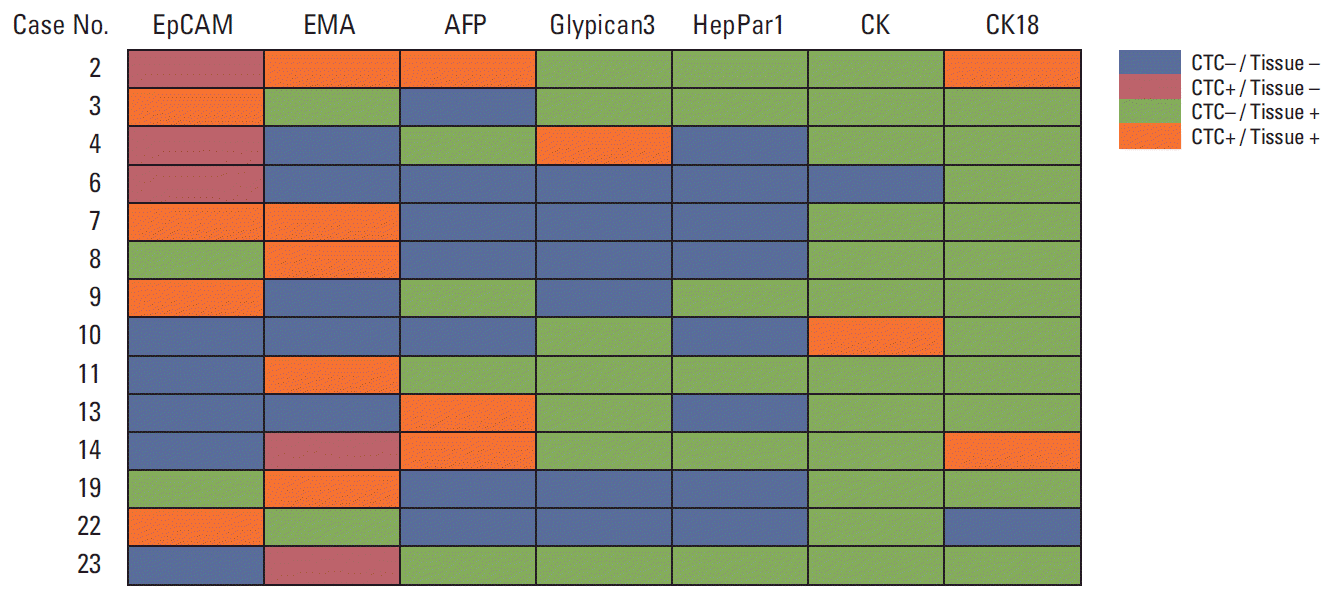
Fig. 5.Survival analysis according to presence of circulating tumor cell (CTC). Kaplan-Meier analyses of survival of patients with liver cancer (A-D) and patients with hepatocellular carcinoma (HCC) (E-H). p-values were determined using log-rank tests. The presence of CTCs and CTCs positive for epithelial cell adhesion molecule (EpCAM) and α-fetoprotein (AFP) resulted in poorer prognosis, but the differences were not statistically significant. EMA, epithelial membrane antigen. 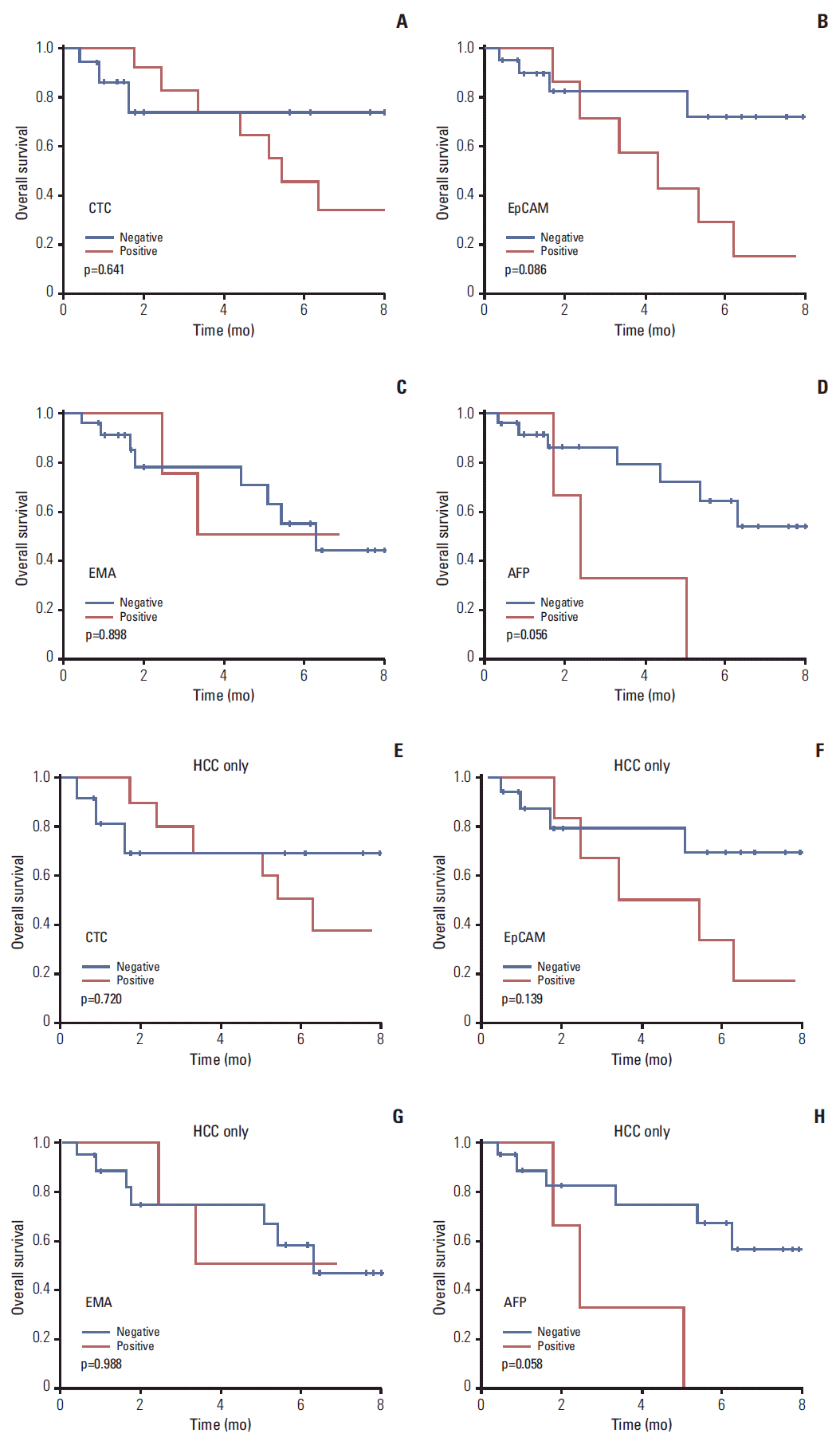
Table 1.Immunohistochemical assays of HCC markers in cell blocks of human liver cancer cell lines spiked to normal peripheral blood
Table 2.Clinicopathologic features of study subjects
Table 3.Expression pattern of CTC markers in CTC-positive patients
Table 4.Comparative analysis of marker expression profiles in CTC and tissue samples from patients with HCC References1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
2. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35:2155–66.
3. Arnaoutakis DJ, Mavros MN, Shen F, Alexandrescu S, Firoozmand A, Popescu I, et al. Recurrence patterns and prognostic factors in patients with hepatocellular carcinoma in noncirrhotic liver: a multi-institutional analysis. Ann Surg Oncol. 2014;21:147–54.
4. Sumie S, Nakashima O, Okuda K, Kuromatsu R, Kawaguchi A, Nakano M, et al. The significance of classifying microvascular invasion in patients with hepatocellular carcinoma. Ann Surg Oncol. 2014;21:1002–9.
5. Harouaka R, Kang Z, Zheng SY, Cao L. Circulating tumor cells: advances in isolation and analysis, and challenges for clinical applications. Pharmacol Ther. 2014;141:209–21.
6. Hyun KA, Kwon K, Han H, Kim SI, Jung HI. Microfluidic flow fractionation device for label-free isolation of circulating tumor cells (CTCs) from breast cancer patients. Biosens Bioelectron. 2013;40:206–12.
7. Grover PK, Cummins AG, Price TJ, Roberts-Thomson IC, Hardingham JE. Circulating tumour cells: the evolving concept and the inadequacy of their enrichment by EpCAM-based methodology for basic and clinical cancer research. Ann Oncol. 2014;25:1506–16.
8. Patriarca C, Macchi RM, Marschner AK, Mellstedt H. Epithelial cell adhesion molecule expression (CD326) in cancer: a short review. Cancer Treat Rev. 2012;38:68–75.
9. Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–9.
10. Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35:122–8.
11. Park H, Lee H, Seo AN, Cho JY, Choi YR, Yoon YS, et al. SALL4 expression in hepatocellular carcinomas is associated with EpCAM-positivity and a poor prognosis. J Pathol Transl Med. 2015;49:373–81.
12. Hewitt RE, McMarlin A, Kleiner D, Wersto R, Martin P, Tsokos M, et al. Validation of a model of colon cancer progression. J Pathol. 2000;192:446–54.
13. Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO classification of tumours of the digestive system. 4th edLyon: IARC Press; 2010.
14. Compton CC, Byrd DR, Garcia-Aguilar J, Kurtzman SH, Olawaiye A, Washington MK. AJCC cancer staging atlas: a companion to the seventh editions of the AJCC cancer staging manual and handbook. 2nd edNew York: Springer; 2012.
15. Vona G, Sabile A, Louha M, Sitruk V, Romana S, Schutze K, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulatingtumor cells. Am J Pathol. 2000;156:57–63.
16. Zieglschmid V, Hollmann C, Bocher O. Detection of disseminated tumor cells in peripheral blood. Crit Rev Clin Lab Sci. 2005;42:155–96.
17. Rolle A, Gunzel R, Pachmann U, Willen B, Hoffken K, Pachmann K. Increase in number of circulating disseminated epithelial cells after surgery for non-small cell lung cancer monitored by MAINTRAC(R) is a predictor for relapse: a preliminary report. World J Surg Oncol. 2005;3:18.
18. Chiappini F. Circulating tumor cells measurements in hepatocellular carcinoma. Int J Hepatol. 2012;2012:684802.
19. Xu W, Cao L, Chen L, Li J, Zhang XF, Qian HH, et al. Isolation of circulating tumor cells in patients with hepatocellular carcinoma using a novel cell separation strategy. Clin Cancer Res. 2011;17:3783–93.
20. Fan ST, Yang ZF, Ho DW, Ng MN, Yu WC, Wong J. Prediction of posthepatectomy recurrence of hepatocellular carcinoma by circulating cancer stem cells: a prospective study. Ann Surg. 2011;254:569–76.
21. Ignatiadis M, Lee M, Jeffrey SS. Circulating tumor cells and circulating tumor DNA: challenges and opportunities on the path to clinical utility. Clin Cancer Res. 2015;21:4786–800.
22. Schulze K, Gasch C, Staufer K, Nashan B, Lohse AW, Pantel K, et al. Presence of EpCAM-positive circulating tumor cells as biomarker for systemic disease strongly correlates to survival in patients with hepatocellular carcinoma. Int J Cancer. 2013;133:2165–71.
23. Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, et al. Circulating stem cell-like epithelial cell adhesion molecule-positive tumor cells indicate poor prognosis of hepatocellular carcinoma after curative resection. Hepatology. 2013;57:1458–68.
24. Ruck P, Wichert G, Handgretinger R, Kaiserling E. Ep-CAM in malignant liver tumours. J Pathol. 2000;191:102–3.
25. de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol. 1999;188:201–6.
26. Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumorinitiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–24.
27. Terris B, Cavard C, Perret C. EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. J Hepatol. 2010;52:280–1.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||