Introduction
Chondrosarcoma is the second most common malignant tumor of bone and is characterized by tumor cells producing cartilage matrix. Chondrosarcoma is a rare cancer with an estimated incidence of 1 in 200,000 per year [1]. A variety of histological types with diverse clinical behavior have been described, with conventional types being the most common. Chondrosarcoma can affect any part of the skeleton but has a predilection for pelvis, femur and proximal humerus [2,3]. Surgery remains the mainstay of treatment as neither chemotherapy nor radiation therapy is effective in chondrosarcoma [4].
The prognostic factors of chondrosarcoma remain uncertain as only a few large studies with long-term follow-up have been reported [5-7]. Identifying prognostic factors is of paramount importance, not only to predict patients’ outcome, but also to guide decisions regarding treatment. In this regard, this study was undertaken in 150 consecutive patients treated in a single institution with a median follow-up of 8.5 years. The aims of this study were to evaluate oncologic outcomes of chondrosarcoma and to identify prognostic factors.
Materials and Methods
From the prospectively collected database of our institute, 150 consecutive patients treated surgically for chondrosarcoma from 1982 to 2011 were retrospectively reviewed. Patient data was retrieved from the electronic medical record system and the study was approved by Institutional Review Board. Among these 150 patients, patients with metastasis at diagnosis (n=13), with myxoid type (n=7), and with insufficient medical records (n=5) were excluded, which left 125 patients for analysis. The histologic types included conventional (n=93), secondary (n=11), dedifferentiated (n=8), clear cell (n=5), periosteal (n=4), and mesenchymal (n=4). The mean follow-up of the 125 patients was 9.1±5.9 years (range, 1.2 to 31.0 years). There were 59 men and 66 women with a median age of 50 years (range, 14 to 77 years).
For analysis of prognostic factors for oncologic outcome, patients with primary central conventional chondrosarcoma (n=93) were selected to construct a homogenous group. Medical records were reviewed for the prognostic factors that might influence oncologic outcome with regard to patient demographics, tumors characteristics and treatment factors.
The mean follow-up was 8.4±5.3 years (range, 1.7 to 21.6 years). There were 37 men and 56 women with a median age of 50 years (range, 14 to 77 years). For the purpose of analysis, patients were dichotomized into groups of age ≤ 50 years or age > 50 years. Sixty-eight tumors were located in the appendicular skeleton and 25 in the axial skeleton, which included pelvis, sacrum and scapula. Tumor volume was calculated in mL based on magnetic resonance imaging (MRI) [8]. In patients where MRI was not available, the tumor size was recorded from histopathology specimen. Patients were dichotomized into two groups of tumor volume ≤ 100 mL or > 100 mL for analysis. Histopathology reports were reviewed to note cellular atypia, mitotic count to grade, size of excised tumor specimen, and pathological margins. There were 62 grade 1 tumors, 21 grade 2 tumors, and 10 grade 3 tumors. For the purpose of analysis, histologic grade was dichotomized into low grade (grade 1, n=62) and high grade (grade 2 or 3, n=31) (Table 1).
In all, 48 tumors were treated with intralesional curettage and 45 tumors with resection. Of the 48 tumors that underwent intralesional curettage, 42 were low-grade tumors of the appendicular skeleton. Among the 45 tumors that underwent resection, 40 were resected with a wide margin and 5 with a marginal margin [9]. Histological negative margins were achieved in 37 of the 45 patients that underwent resection. Adjuvant treatment plans were discussed in interdisciplinary meetings although no prospectively defined protocol was followed. Chemotherapy was administered in four patients and radiotherapy with the median dose of 47 Gy (range, 42 to 50 Gy) was given postoperatively in six patients.
Patients were followed up regularly at 3-month intervals for the initial 2 years, then at 6-month intervals for 3 years and later annually. For surveillance for metastasis, chest computed tomography and bone scans were performed. Imaging of the primary site was done with plain radiographs, MRI or ultrasound based on the risk of local recurrence (LR).
The oncologic outcomes of this study were disease-specific death, metastasis, and LR. Disease-specific survival (DSS) was measured from the date of surgery to the date of the patient’s death. Patients who were alive or died of other causes were classified as censored observations at the time of the last follow-up. Metastasis-free survival was calculated from the date of surgery to the date when metastasis was first recorded. Local recurrence-free survival (LRFS) was calculated from the date of surgery to the date when LR was first recorded. Various clinical-pathologic factors were analyzed for prognostic effect on DSS and LRFS for central type chondrosarcoma. Survival was estimated using Kaplan-Meier survival curves and the log-rank test for univariate analysis. To adjust for confounders, multivariate analysis using the Cox proportional hazards model was performed using the variables with p-values of < 0.05 in univariate analysis. A p-value of less than 0.05 was considered significant. Statistical analyses were performed using the IBM SPSS ver. 21.0 (IBM Co., Armonk, NY).
Results
1. Survival according to histologic types
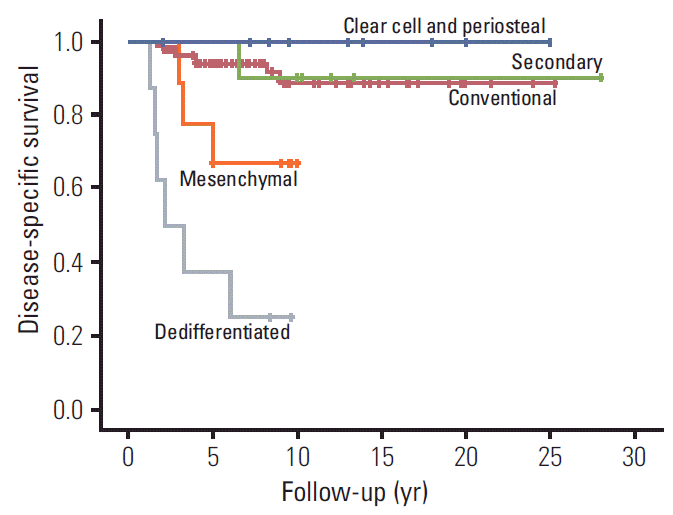
For the entire cohort of 125 patients encompassing all histological types, 17 patients (14%) died of disease and two patients (2%) died of other causes. One hundred and six patients were alive, of whom 100 were disease-free and six were alive with disease. DSS of 125 patients were 91.6%±2.5%, 84.1%±3.8%, and 84.1%±3.8% at 5, 10, and 15 years, respectively. DSS differed significantly among histologic types as dedifferentiated types showed the worst survival (25.0%±15.3% at 10 years) while clear cell and periosteal types showed the best survival (100% at 10 years; p < 0.001) (Fig. 1).
2. Prognostic factors of conventional chondrosarcoma
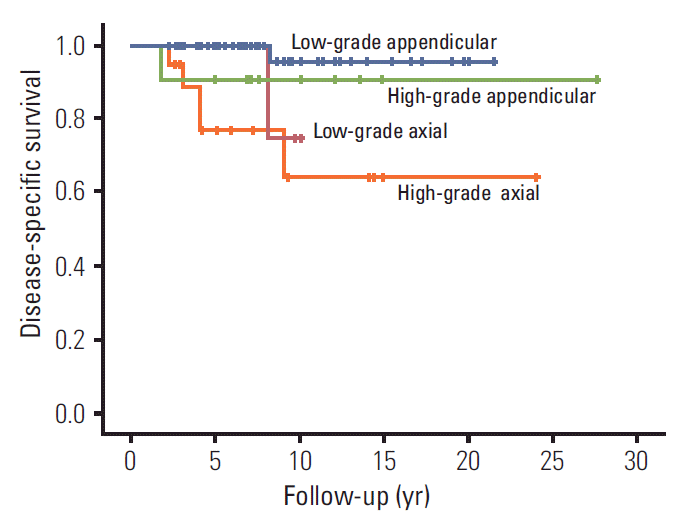
For conventional type chondrosarcoma (n=93), prognostic factors for DSS, metastasis and LR were analyzed. Of the 93 patients, eight patients died of disease before the last follow-up. DSS for conventional type chondrosarcoma was 94%±2.6% and 89.3%±4.1% at 5 and 10 years, respectively. On univariate analysis, high histological grade (p=0.006) and axial location (p=0.010) were associated with the worse outcome (Table 2). Combining these two factors showed significant separation on Kaplan-Meier survival curve analyses (p=0.009) (Fig. 2).
Metastasis was identified in nine out of 93 patients. The median time to metastasis was 5.0 years (range, 0.8 to 23.0 years). On univariate analysis, high histological grade (p=0.006) and axial location (p=0.011) were associated with worse outcome (Table 3). Among the nine patients, four patients underwent metastatectomy, one of whom died of disease.
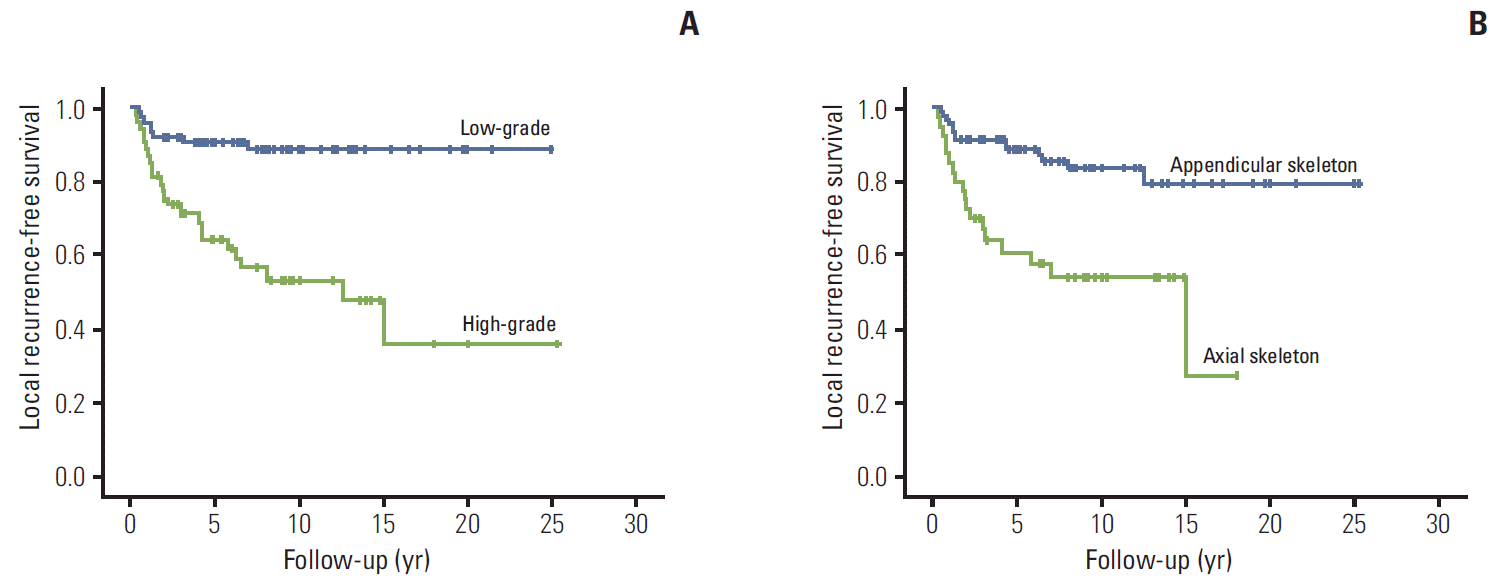
Of the 93 patients with conventional type chondrosarcoma, LR developed in 19 patients and the median time to LR was 6.5 years (range, 0.5 to 15.0 years) (high grade 15, low grade 4; axial skeleton 15, appendicular skeleton 7). Six patients had more than one episode of LR during the course of treatment. LRFS was 80.9%±4.2%, 78.7%±4.6%, and 70.8%±8.5% at 5, 10, and 15 years, respectively. On univariate analysis, high histologic grade (p < 0.001), axial location (p=0.008), and male sex (p=0.010) were associated with worse LRFS. On multivariate analysis, high histologic grade remained an independent prognostic factor for poor LRFS (p=0.012; hazard ratio, 5.32; 95% confidence interval, 0.05 to 0.693) (Fig. 3). Tumor size, surgical margins, and pathological margins did not affect LRFS (Table 4). All patients with LR underwent excision.
Discussion
This study describes the long-term outcomes of 125 patients with chondrosarcoma treated at a single institution. We believe this is the largest series of chondrosarcoma from a single institution in Korea. Survival in chondrosarcoma has remained unchanged over last 30 years with no significant improvement as emphasized in Surveillance, Epidemiology, and End Results (SEER) database study [1]. This study sought to examine the oncologic outcomes and to identify prognostic factors of chondrosarcoma.
Histological type had significant bearing on the oncologic outcome of chondrosarcoma, as dedifferentiated types had the worst outcome while clear cell types had the best outcome. For the conventional type chondrosarcoma, histological grade and anatomical location were the two most significant factors that predicted different oncologic endpoints, namely disease-specific death, metastasis and LR in line with previous studies [1,4,7,10,11].
Chondrosarcoma of axial skeleton showed poor oncologic outcomes compared to appendicular skeleton consistent with previous studies [1,4,10,12-16]. Of note, the majority of conventional chondrosarcomas of axial skeleton were high-grade tumors (20 of 25). Whether this observation is due to a tumor microenvironment advantageous for tumor progression or the late diagnosis inherent to the anatomical confines remains to be elucidated. Among the five low-grade tumors of axial skeleton, there were no deaths and one LR.
Of the 57 patients with low-grade chondrosarcomas of appendicular skeleton, only one patient died of disease. Moreover, only three LRs was observed among the 43 cases with intralesional curettage. These findings may reflect the benign nature of low-grade chondrosarcomas of appendicular skeleton [17]. In addition, the relatively larger proportion (67%) of low-grade chondrosarcomas in this study might explain the high survival and local control rate of this study. The one patient who died of low-grade chondrosarcoma of appendicular skeleton had a tumor of the distal femur with extraosseous extension. This case illustrates that the Enneking stage IB chondrosarcomas have the potential for systemic spread and should thus be treated carefully.
Male gender was a risk of LR in univariate analysis in line with the previous report [1]. This finding could not be explained by the difference in the proportion of axial chondrosarcomas as previously noted [1], as the proportion of axial chondrosarcomas were not different between males and females (11/38 vs. 14/55, p=0.813). However, the incidence of LR in axial tumors was significantly higher in male patients compared to that of female patients (8/11 vs. 4/14, p=0.047). Whether this finding is due to the difference in anatomical complexity between genders or the inherent biological aggressiveness of the tumor remains to be seen.
Some of the well-established prognostic factors of chondrosarcoma, such as tumor size or surgical margin, did not influence DSS or LRFS in this study of chondrosarcoma [7,10,14,18-20]. Inclusion of low-grade appendicular chondrosarcomas, which consistently show good prognosis regardless of known prognostic factors, may have contributed to this finding. Moreover, the limited number of patients limits the interpretation of the prognostic value of age or surgical margin.
The development of LR did not always result in patient death as 15 out of 19 patients were alive at the last follow-up. Moreover, metastasis developed in only five of 19 patients with LR. These findings suggest that local failure is not always associated with systemic progression. However, the oncological significance of LR seems to differ among cases and better indicators are needed. Of note, all patients with LR underwent excision of the recurred tumor.
This study has a few limitations. First, the retrospective nature of this study limits the interpretation of the results. However, the rarity of chondrosarcomas makes it difficult to overcome. Second, treatment may not have been uniform as this study was conducted over a long period of time. However, the therapeutic approach has remained largely unchanged for chondrosarcoma [1]. Third, the effect of adjuvant therapy on oncologic outcomes could not be examined due to the limited number of patients.