AbstractPurposeThe purpose of this study was to compare ramosetron (RAM), aprepitant (APR), and dexamethasone (DEX) [RAD] with palonosetron (PAL), APR, and DEX [PAD] in controlling highly-emetogenic chemotherapy (HEC)–induced nausea and vomiting.
Materials and MethodsPatients were randomly assigned (1:1) to receive RAD or PAD:RAM (0.3 mg intravenously) or PAL (0.25 mg intravenously) D1, combined with APR (125 mg orally, D1 and 80 mg orally, D2-3) and DEX (12 mg orally or intravenously, D1 and 8 mg orally, D2-4). Patients were stratified by sex, cisplatin-based chemotherapy, and administration schedule. The primary endpoint was overall complete response (CR), defined as no emesis and no rescue regimen during 5 days of HEC. Secondary endpoints were overall complete protection (CP; CR+nausea score < 25 mm) and total control (TC; CR+nausea score < 5 mm). Quality of life was assessed by Functional Living Index Emesis (FLIE) questionnaire on D0 and D6.
ResultsA total of 279 patients receiving RAD (n=137) or PAD (n=142) were evaluated. Overall CR rates in RAD and PAD recipients were 81.8% and 79.6% (risk difference [RD], 2.2%; 95% confidence interval [CI], −7.1 to 11.4), respectively. Overall CP and TC rates for RAD and PAD were 56.2% and 58.5% (RD, −2.3%; 95% CI, −13.9 to 9.4) and 47.5% vs. 43.7% (RD, 3.8%; 95% CI, −7.9 to 15.5), respectively. FLIE total score ≥ 108 (no impact on daily life) was comparable between RAD and PAD (73.9% vs. 73.4%, respectively). Adverse events were similar between the two groups.
IntroductionChemotherapy-induced nausea and vomiting (CINV) has been reported as the fifth most feared symptom in cancer patients receiving systemic cytotoxic chemotherapy [1,2]. Overwhelming evidence shows that CINV, when not adequately controlled, degrades the quality of life (QOL) of cancer patients. Although CINV is feared by cancer patients receiving highly-emetogenic chemotherapy (HEC), it can be controlled [2,3].
Based on phase III study data, the triple-drug combination of ondansetron (OND), dexamethasone (DEX), and aprepitant (APR) was regarded as the standard antiemetic regimen for cancer patients receiving HEC [4]. Palonosetron (PAL) has a longer half-life and stronger 5HT3 receptor binding affinity than other 5HT3 receptor antagonists (5HT3RAs), including OND, granisetron (GRA), and dolasetron (DOL) [5]. Moreover, PAL alone or in combination with DEX exhibited better antiemetic activity than GRA or DOL combined with DEX for the control of acute and delayed CINV in patients receiving moderately-emetogenic chemotherapy (MEC) [6,7]. National comprehensive cancer network guidelines currently recommend the combination of PAL, APR, and DEX (PAD) as a standard regimen for controlling HEC-induced CINV in cancer patients [8]. However, there are no direct head-to-head comparisons of PAD vs. other 5HT3RAs.
Ramosetron (RAM), a 5HT3RA with a prolonged half-life and increased receptor binding affinity compared to OND or GRA [9], has been used widely for CINV prevention in Asia. The antiemetic activity of RAM was shown to be similar to that of PAL for controlling MEC-induced CINV [10].
The purpose of this phase IV study was to show the non-inferiority of the combination of RAM, APR, and DEX (RAD) compared to PAD in controlling HEC-induced CINV.
Materials and Methods1. Study designThis prospective, multicenter, single-blind, randomized phase IV clinical trial was conducted in 10 institutions. Patients were randomly assigned to RAD or PAD (1:1 ratio). The study was conducted under single-blind conditions, with participants not being aware of their assignment group.
Inclusion criteria included age 19-75 years, pathologically-confirmed malignant disease, and Eastern Cooperative Oncology Group performance status 0-2. Patients were scheduled to receive HEC on the first day of treatment. Patients were required to have adequate bone marrow, hepatic, and renal function. Major exclusion criteria included medications, medical illness, or medical conditions and procedures that could affect nausea or vomiting (a complete list of eligibility criteria is provided in the S1 Table).
2. Study treatmentAPR (125 mg orally, 1 hour prior to chemotherapy, D1; 80 mg orally, D2-3) and DEX (12 mg, orally or intravenously, 30 minutes prior to chemotherapy, D1; 8 mg, orally, D2-4) were administered in both arms. The RAM and PAL arms received RAM (0.3 mg, D1) or PAL (0.25 mg, D1) intravenously 30 minutes before chemotherapy. Rescue antiemetics for severe nausea/vomiting were administered at the request of the patient or upon recommendation by the attending physicians at any time during the study period.
3. Study endpointsThe primary endpoint was overall complete response (CR), defined as no vomiting, including retching, and no requirement for rescue antiemetics within 5 days of HEC. Secondary endpoints were CR, complete protection (CP; CR+nausea score < 25 mm; 0-100 mm), and total control (TC; CR+nausea score < 5 mm; 0-100 mm) in the acute (0-24 hours), delayed (D2-5), and overall (D0-5) periods; severity of nausea (determined using a 0-100 mm visual analog scale); time to first occurrence of vomiting; QOL assessed by the validated patient self-assessment Functional Living Index Emesis (FLIE) questionnaire [11]; safety. During the overall period, patients recorded daily episodes of vomiting or retching, the degree of nausea, and the use of rescue medication in a diary and using the Rhodes Index of Nausea and Vomiting Form-2 (INV-2) [12]. Safety was evaluated based on clinical and laboratory adverse events (AEs) between the start day and the day before the next chemotherapy, assessed according to National Cancer Institute–Common Terminology Criteria for Adverse Events (NCI-CTCAE) ver. 4.03 [13].
4. FLIE measurement and scoringFLIE includes 18 questions divided into two domains: nausea (questions 1-9) and vomiting (questions 10-18) [11]. The questionnaire was completed on D0 (reflecting daily-life activities before chemotherapy) and D6 (to provide information on the overall effect of CINV on daily-life activities during D1-5). The average score for each domain was summed and transformed using a pre-specified scoring procedure, which allowed for a minimum domain score of 6 and a maximum of 63. The chi-square test was used to compare the proportion of patients treated with RAM vs. PAL, with ‘no impact on daily life’ (NIDL) (i.e., individual question scores > 6 on the 7-point FLIE scale, domain score ≥ 54, overall FLIE score≥ 108) assessed for the FLIE domains of nausea, vomiting, and combined (i.e., total score).
5. Statistical analysisThe hypothesis was that the CR rate in the RAD arm during the overall period would not be inferior to the CR rate in the PAD arm. A sample size of 135 in each arm allowed an 80% power and one-sided α level of 0.025 to detect a CR non-inferiority difference of −15% between the RAD and PAD groups, assuming the actual CR in each group to be 77% and 77%, respectively. With an expected dropout rate of 15%, sample size was increased to 146 patients per arm.
Stratified block randomization was conducted with a 1:1 ratio between groups, randomly mixing block sizes of 2 and 4, considering (1) chemotherapeutic regimen (cisplatin vs. non-cisplatin), (2) treatment schedule (single-day vs. multiple-day), and (3) sex (male vs. female), as stratification factors. Patients were assigned according to a pre-defined randomization sequence created by an independent investigator with no clinical involvement in the trial.
Effectiveness was calculated in the modified intention-to-treat population, defined as all patients randomly assigned to a treatment group having received at least one study treatment dose after randomization. Data were analyzed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC). The two groups were compared using the chi-square test, Fisher exact test, and Wilcoxon rank-sum test, as appropriate.
6. Ethical statementThe study protocol was approved by the institutional review boards at the Seoul Saint Mary’s Hospital (KC15MIMV0785), Kangdong Sacred Heart Hospital (KANGDONG-2015-05-005), Kangbuk Samsung Hospital (KBSMC 2015-03-010), Keimyung University Dongsan Hospital (DSMC 2015-06-060), St. Vincent's Hospital (VC16MIMV0059), Severance Hospital, Yonsei Cancer Center (4-2015-0414), Pusan National University Hospital (H-1506-003-044), Ajou University Hospital (AJIRB-MED-CT4-15-083), Chungnam National University Hospital (CNUH 2015-02-025), Samsung Medical Center (SMC-2015-01-096) and registered with ClinicalTrials.gov (NCT02532634); all patients provided written informed consent.
Results1. PatientsBetween August 2015 and September 2017, a total of 309 patients were screened and 292 eligible patients were randomly assigned (1:1) to receive RAD or PAD (Fig. 1). Patient characteristics, disease demographics, chemotherapeutic regimens, and administration schedules were well balanced between both arms (Table 1).
2. EfficacyThe overall CR rates of RAD and PAD were 81.8% and 79.6% (risk difference [RD], 2.2%; 95% confidence interval [CI], −7.1 to 11.4). RAD was non-inferior to PAD, as evidenced by CR rates in the acute, delayed, and overall periods (RD, 2.0%, 4.4%, and 2.2%, respectively) (Table 2, Fig. 2A).
3. Quality of lifeThe total FLIE score with RAD (115.5±17.6) was comparable to that with PAD (114.8±16.7, p=0.745). The proportion of patients with FLIE score ≥ 108 (NIDL) in RAD (75.0%) was comparable to that in PAD (73.9%) (RD, 1.09; 95% CI, −9.32 to 11.49; p=0.838). The proportion of patients with FLIE score ≥ 54 in each of the nausea and vomiting domains in RAD was numerically higher than in PAD (72.2% vs. 65.9% and 91.2% vs. 91.3%, respectively), but not statistically significant (p=0.267 and p=0.565, respectively) (Table 3).
4. Adverse eventsA total of 292 patients receiving RAD or PAD were evaluated in the safety cohort. Overall, no new safety signals were identified. Both RAD and PAD were well tolerated, with > 90% of AEs mild or moderate (Table 4). The most common hematologic and non-hematologic toxicities were neutropenia and anorexia, respectively.
DiscussionPAD is the standard regimen for controlling CINV in cancer patients receiving HEC. We conducted a prospective, multicenter, single-blind, randomized clinical study to prove the non-inferiority of RAD vs. PAD for the control of HEC-induced CINV.
The distribution of patients between both treatment groups was well balanced by patient-related and chemotherapy-related factors. The primary endpoint of the study was overall CR throughout 5 days post-HEC. The CR rate in PAD recipients, used as the reference arm, was comparable to rates in 1,453 patients who were randomized to PAD in seven randomized controlled trials (RCTs) for HEC-induced CINV [14-20]; CR rates in the RCTs vs. our study were 86.6% vs. 93.7%, 73.4% vs. 79.6%, and 69.5% vs. 79.6% in the acute, delayed, and overall phases, respectively (Table 5). Meanwhile, the overall CR rate (81.8%) with RAD was slightly higher than in our previous phase III study (77.1%) [21], but comparable to that with PAD (79.6%). QOL with FLIE was very similar between RAD and PAD in terms of nausea, vomiting, and total score. In addition, the proportion of patients with FLIE score ≥ 108 (NIDL) in RAD was comparable to that in PAD. These results strongly support the hypothesis that RAD is non-inferior to PAD, as evidenced by FLIE score as well as CR rates in the acute, delayed, and overall phases.
CR, CP, and TC in RAD did not differ from those in PAD. The number of patients with a FLIE score in the nausea domain ≥ 54 was also higher with RAD than PAD although it was not statistically significant. Considering that the definition of CP allows a low level of nausea, unlike TC, the mechanism of action of RAM may be different from PAL. Previously, Japanese researchers reported that RAM had higher affinity for cloned human and rat 5HT3 receptors, as well as extremely slower dissociation from the human 5HT3 receptor compared with other 5-HT3RAs including alosetron and cilansetron [22]. Accordingly, there are two possible explanations about the conflicting results in our study: (1) RAM may have stronger binding to 5HT3 receptors present on enterochromaffin cells; (2) RAM may block the extramembrane domain of 5HT3 receptor binding to serotonin more effectively.
Subgroup analyses by age, sex, chemotherapy regimen, and tumor type, proved that RAD is non-inferior to PAD. Although the subgroup analysis is controversial regarding statistical significance due to some results exceeding the non-inferiority margin, RAD and PAD displayed distinct activities regarding different aspects of individual subgroups. Given the fact that cisplatin-containing HEC was administered for all lung cancer patients, RAM seems to be more effective for cisplatin-induced nausea and vomiting. Meanwhile, because non-cisplatin chemotherapy was given to all breast cancer patients, PAL seems to be more effective for non-cisplatin che-motherapy-induced nausea and vomiting.
FLIE scores in the total, nausea, and vomiting domains with RAD were quite similar to those with PAD. Patients with a FLIE total score ≥ 108 (NIDL) and a FLIE score in the vomiting domain ≥ 54 were comparable between both groups, but the number of patients with a FLIE score in the nausea domain ≥ 54 was numerically higher in RAD than PAD. There is a possibility that the higher TC rate with RAD is reflected in a QOL benefit, with a correspondingly greater proportion of patients with NIDL due to more effective nausea control during the overall period. While this difference is small, it is encouraging that RAD has some potential to improve QOL.
In this study, no new safety signals were identified. More than 90% of AEs were mild or moderate in intensity. The incidence and duration of serious AEs or AEs was low and similar between both groups.
There are several study limitations. Firstly, because the study aim was to prove the non-inferiority of RAD vs. PAD, subgroup analysis was not scheduled. Although subgroup analysis produced some degree of differences between both arms, some analysis results did not show statistical significance due to the small number of subjects. Secondly, FLIE questionnaire results were not been collected on day 2, reflecting QOL during the acute period. Delayed emesis negatively influences patient QOL during administration of HEC and beyond. Both 5HT3RAs demonstrated significant inhibition against HEC-induced CINV, but did not improve control of delayed nausea, indicating that unmet needs remain for novel antiemetics.
In conclusion, in all aspects of efficacy, safety, and QOL, our data suggested that RAD was comparable to PAD for controlling HEC-CINV in cancer patients.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Conflicts of InterestJH Kang has acted as an advisor for Amgen, Roche, Merck, MSD, Ono/BMS, AstraZeneca, YooHan, SL Bigen, has received research funding from AstraZeneca, Boehringer Ingelheim, Ono, Yoohan, and ChongKunDang, and has acted as a speaker for AstraZeneca, Roche, Merck, and Boehringer Ingelheim. JH Sohn has received research funding from MSD, Roche, Novartis, AstraZeneca, Lilly, Pfizer, Bayer, GSK, CONTESSA, and Daiichi Sankyo. JS Ahn reports personal fees from Amgen, personal fees from Pfizer, personal fees from AstraZeneca, personal fees from Menarini, personal fees from Roche, personal fees from Eisai, personal fees from Boehringer Ingelheim, personal fees from BMS-Ono, personal fees from MSD, personal fees from Janssen, personal fees from Samsung Bioepis, outside the submitted work. All remaining authors declare no conflicts of interest. AcknowledgmentsThe authors thank the clinical investigators, patients, and their families. They also acknowledge the hard work and great effort of the site clinical research nurses who participated in the study: So Hee Park, Hee Yeon Nam, Ji Yun Won, Su Jung Han, Haejeung Kim, Yujeoung Park, Ehyun Choi, Miseon Yu, Da Yeon Gwak, So Ra Jeong, Yang Soon Kim, Hwa Young Lee, Seryun Kang, Eun Hee Cho, Eun Joo Hong, Jin Joo Hong. Editorial support was provided, under the guidance of the authors, by David P. Figgitt PhD, ISMPP CMPP™, Content Ed Net, with funding from Astellas Pharma Korea, Inc.
Fig. 1.Complete response rate (A), complete protection rate (B), and total control rate (C) in intention-to-treat population (n=279) on a daily basis. (A) The risk difference between the two arms was 2.2% (95% confidence interval [CI], –7.1% to 11.4%). (B) The risk difference between the two arms was –2.3% (95% CI, –13.9% to 9.4%). (C) The risk difference between the two arms was 3.8% (95% CI, –7.9% to 15.5%) using the generalized estimating equation model. HEC, highly-emetogenic chemotherapy; mITT, modified intention to treatment. 
Fig. 2.Complete response rate (A), complete protection rate (B), and total control rate (C) in intention-to-treat population (n=279) on a daily basis. There was no statistical significance by individual date. (A) The risk difference between the two arms was 2.2% (95% confidence interval [CI], –7.1 to 11.4). (B) The risk difference between the two arms was –2.3% (95% CI, –13.9 to 9.4). (C) The risk difference between the two arms was 3.8% (95% CI, –7.9 to 15.5) using the generalized estimating equation model. RAD, ramosetron, aprepitant, and dexamethasone; PAD, palonosetron, aprepitant, and dexamethasone. 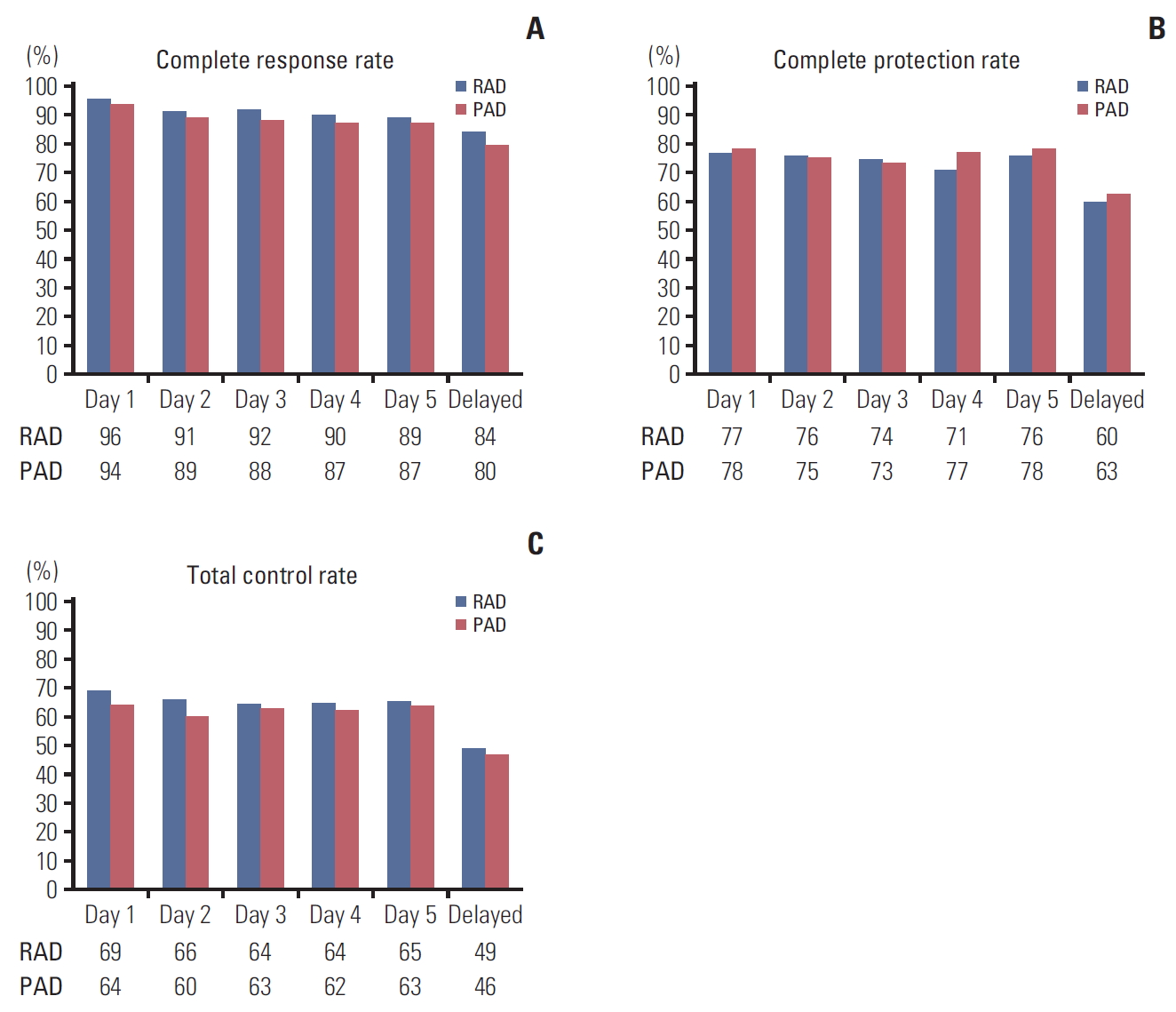
Table 1.Demographics
Values are presented as number (%) or mean±standard deviation. RAD, ramosetron, aprepitant, and dexamethasone; PAD, palonosetron, aprepitant, and dexamethasone; GU, genitourinary; GY, gynecology; Gastrointestinal, esophagus, stomach, colorectum; Others, lymphoma, skin; NA, not available; ECOG, Eastern Cooperative Oncology Group. b) Individual chemotherapy regimens are detailed in S2 Table. Table 2.Complete response, complete protection, and total control rates in intention-to-treat population
Table 3.FLIE score and proportion of patients reporting NIDL for total, nausea domain, and vomiting domain in the mITT population
Table 4.Safety profile (any adverse events with incidence > 1%) Values are presented as number (%). Adverse events were graded according to National Cancer Institute–Common Terminology Criteria (CTCAE) ver. 4.03. RAD, ramosetron, aprepitant, and dexamethasone; PAD, palonosetron, aprepitant, and dexamethasone; AST, aspartate aminotransferase; ALT, alanine aminotransferase. Table 5.Randomized phase III clinical trials of ramosetron or palonosetron in combination with NK-1R antagonists and dexamethasone for control of HEC-induced CINV
HEC, highly-emetogenic chemotherapy; CINV, chemotherapy-induced nausea and vomiting; CR, complete response (no emesis, no rescue medication); CP, complete protection (CR and nausea score < 25 [0-100]); TC, total control (CR and no nausea); CDDP, cisplatin; AC, adriamycin, cyclophosphamide; RAD, ramosetron, aprepitant, dexamethasone; OAD, ondansetron, aprepitant, dexamethasone; PAD, palonosetron, aprepitant, dexamethasone; GAD, granisetron, aprepitant, dexamethasone; OPD, olanzapine, palonosetron, dexamethasone; NEPD, netupitant, palonosetron, dexamethasone; PD, palonosetron, dexamethasone; NEPA300, netupitant 300 mg+palonosetron 0.5 mg; PADM, palonosetron, aprepitant, dexamethasone, metoclopramide; NR, not reported; MEC, moderately-emetogenic chemotherapy. References1. Lindley C, McCune JS, Thomason TE, Lauder D, Sauls A, Adkins S, et al. Perception of chemotherapy side effects cancer versus noncancer patients. Cancer Pract. 1999;7:59–65.
2. Passik SD, Kirsh KL, Rosenfeld B, McDonald MV, Theobald DE. The changeable nature of patients' fears regarding chemotherapy: implications for palliative care. J Pain Symptom Manage. 2001;21:113–20.
3. Fernandez-Ortega P, Caloto MT, Chirveches E, Marquilles R, Francisco JS, Quesada A, et al. Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients' quality of life. Support Care Cancer. 2012;20:3141–8.
4. Hesketh PJ, Grunberg SM, Gralla RJ, Warr DG, Roila F, de Wit R, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21:4112–9.
5. Grunberg SM, Koeller JM. Palonosetron: a unique 5-HT3-receptor antagonist for the prevention of chemotherapy-induced emesis. Expert Opin Pharmacother. 2003;4:2297–303.
6. Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, Cartmell A, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003;98:2473–82.
7. Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, Peschel C, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14:1570–7.
8. National Comprehensive Cancer Network. Antiemesis (version 1.2020) [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2020. [cited 2020 Feb 27]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf
9. Rabasseda X. Ramosetron, a 5-HT3 receptor antagonist for the control of nausea and vomiting. Drugs Today (Barc). 2002;38:75–89.
10. Kim JS, Kim JY, Lee SJ, Park DK, Namgung H, Kim CN, et al. Multicenter nonrandomized trial of ramosetron versus palonosetron in controlling chemotherapy-induced nausea and vomiting for colorectal cancer. Ann Surg Treat Res. 2014;87:9–13.
11. Martin AR, Pearson JD, Cai B, Elmer M, Horgan K, Lindley C. Assessing the impact of chemotherapy-induced nausea and vomiting on patients' daily lives: a modified version of the Functional Living Index-Emesis (FLIE) with 5-day recall. Support Care Cancer. 2003;11:522–7.
12. Rhodes VA. Criteria for assessment of nausea, vomiting, and retching. Oncol Nurs Forum. 1997;24(7 Suppl):13–9.
13. . National Cancer Institute-Common Terminology Criteria for Adverse Events (NCI-CTCAE) [Internet]. Bethesda, MD: National Cancer Institute; 2010. [cited 2019 Sep 11]. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
14. Suzuki K, Yamanaka T, Hashimoto H, Shimada Y, Arata K, Matsui R, et al. Randomized, double-blind, phase III trial of palonosetron versus granisetron in the triplet regimen for preventing chemotherapy-induced nausea and vomiting after highly emetogenic chemotherapy: TRIPLE study. Ann Oncol. 2016;27:1601–6.
15. Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase III trial. J Support Oncol. 2011;9:188–95.
16. Hesketh PJ, Rossi G, Rizzi G, Palmas M, Alyasova A, Bondarenko I, et al. Efficacy and safety of NEPA, an oral combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy: a randomized dose-ranging pivotal study. Ann Oncol. 2014;25:1340–6.
17. Roila F, Ruggeri B, Ballatori E, Fatigoni S, Caserta C, Licitra L, et al. Aprepitant versus metoclopramide, both combined with dexamethasone, for the prevention of cisplatin-induced delayed emesis: a randomized, double-blind study. Ann Oncol. 2015;26:1248–53.
18. Gralla RJ, Bosnjak SM, Hontsa A, Balser C, Rizzi G, Rossi G, et al. A phase III study evaluating the safety and efficacy of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting over repeated cycles of chemotherapy. Ann Oncol. 2014;25:1333–9.
19. Wu F, Lin X, Yang Z, Sun Z, Zeng F, Heng J, et al. Phase III randomized trial of palonosetron and dexamethasone with or without aprepitant to prevent nausea and vomiting induced by full-dose single-day cisplatin-based chemotherapy in lung cancer. Clin Lung Cancer. 2018;19:e913–8.
20. Zhang L, Lu S, Feng J, Dechaphunkul A, Chang J, Wang D, et al. A randomized phase III study evaluating the efficacy of single-dose NEPA, a fixed antiemetic combination of netupitant and palonosetron, versus an aprepitant regimen for prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC). Ann Oncol. 2018;29:452–8.
21. Kim HJ, Shin SW, Song EK, Lee NR, Kim JS, Ahn JS, et al. Ramosetron versus ondansetron in combination with aprepitant and dexamethasone for the prevention of highly emetogenic chemotherapy-induced nausea and vomiting: a multicenter, randomized phase III trial, KCSG PC10-21. Oncologist. 2015;20:1440–7.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||