Multiple Cardiac Metastases from a Nonfunctioning Pancreatic Neuroendocrine Tumor
Article information
Abstract
Pancreatic neuroendocrine tumors (pNETs) are rare neoplasms, which most commonly metastasize to the liver. However, intrathoracic metastases from pNETs are encountered infrequently. This report describes a case of nonfunctioning pNET with multiple cardiac metastases. A 56-year-old male presented with a palpable abdominal mass that showed progressive enlargement. Findings on computed tomography (CT) of the abdomen revealed two relatively well-marginated inhomogeneous low-attenuation masses, one in the head of the pancreas and the other in the tail. Multiple enhancing masses in the left pericardium with myocardial involvement were observed on chest CT and transthoracic echocardiography. Needle biopsies were performed on the mass in the tail of the pancreas and the left ventricular apical pericardium; histologic examination by hematoxylin and eosin morphology and immunohistochemical staining showed pNET in both. This is the first report of pNET with multiple cardiac metastases to previously undescribed metastatic sites.
Introduction
Pancreatic neuroendocrine tumors (pNETs) are low- to intermediate-grade neoplasms, which are considered to arise from pancreatic islets and are relatively rare. The incidence and prevalence of metastatic pNETs are increasing, and currently represent 1.3% of all cases of pancreatic cancer in incidence and 10% of cases in prevalence [1-3]. pNETs are classified as functional (10-30% of tumors) or nonfunctional (50-80%) [4]. Functional pNETs are usually associated with a hormonal syndrome due to hormone-excess. By contrast, all symptoms of nonfunctional pNETs are associated with the tumor per se. Therefore, some small or undetectable primary functional pNETs may present with severe symptoms, whereas nonfunctional pNETs almost always present late in the disease course with large tumors that are usually metastatic [4,5]. The most common sites of metastases include liver, lymph nodes, and bone [4]. Intrathoracic metastases from pNETs are encountered infrequently; however, cardiac metastasis has not been previously reported. This report describes a case of nonfunctioning pNET with cardiac metastases.
Case Report
A 56-year-old male presented to the clinic in December 2008, with a two-week history of a progressively enlarging palpable mass in the left lower abdomen, abdominal pain, and dyspepsia. He had no relevant medical history. Findings on physical examination revealed the following: body temperature, 36.8℃; blood pressure, 130/75 mm Hg; and pulse rate, 60 per minute. Heart sounds were normal, without murmur, rubs, or gallops. However, on abdominal examination, a firm, fixed mass measuring 7 cm in size, without tenderness or rebound tenderness, was found in the left lower abdomen. After extensive history taking and physical examination, no clinical symptoms or signs of a hormone-excess were observed. Results of laboratory tests were as follows: hemoglobin, 13.0 g/dL; white cell count, 4,000/µL; and platelet count, 196,000/µL. Blood chemistry findings were as follows: alanine aminotransferase, 23 IU/L; aspartate aminotransferase, 12 IU/L; total bilirubin, 0.3 mg/dL; and alkaline phosphatase, 132 IU/L. Levels of serum tumor markers were as follows: carcinoembryonic antigen (CEA), 21.19 U/mL; cancer antigen 19-9, 1.51 ng/mL; and neuron-specific enolase, 9.75 ng/mL. Hormone levels were within the normal range (gastrin, 162.0 pg/mL; fasting insulin, 21.4 µU/mL; glucagon, 83.4 pg/mL; somatostatin, 8.8 pg/mL; and corticotrophin, 58.1 pg/mL). A 12-lead electrocardiography showed sinus bradycardia (ventricular rate, 53 beats per minute) with nonspecific ST segment and T wave changes. Findings on chest radiography were normal. Findings on computed tomography (CT) of the abdomen revealed two relatively well-marginated inhomogeneous low-attenuation masses, one measuring 7.3×8.8 cm in the head of the pancreas and the other measuring 12.0×9.2 cm in the tail (Fig. 1), with multiple enlarged lymph nodes in the peripancreatic area. A CT scan of the pelvis showed bone destruction in the left pelvic bone. A chest CT scan showed multiple enhancing heterogeneous masses distributed in the left pericardium (Fig. 2). Transthoracic echocardiography showed multiple areas of well-circumscribed echogenic masses in the left pericardium, with myocardial involvement (Fig. 3), but with preserved left ventricular function. No associated valvular abnormality was observed, and no intracardiac shunt was detected by contrast study.
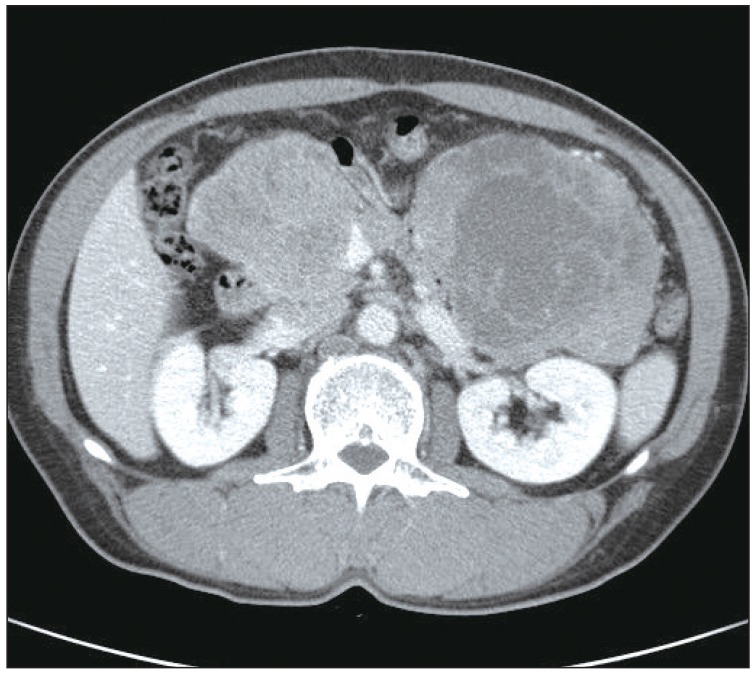
Computed tomography (CT) of the abdomen. CT of the abdomen showed well-marginated enhancing large masses, one measuring 7.3×8.8 cm in the head of the pancreas and one measuring 12.0×9.2 cm in the tail.
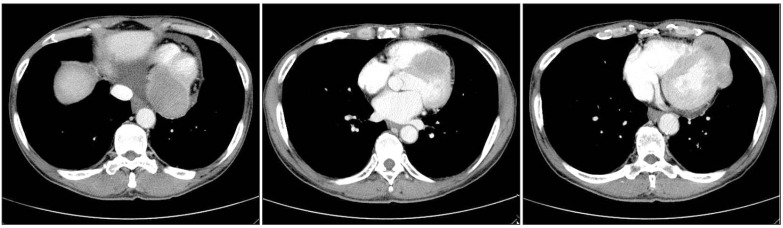
Computed tomography (CT) of the chest. Chest CT showed multiple enhancing masses distributed in the left pericardium.
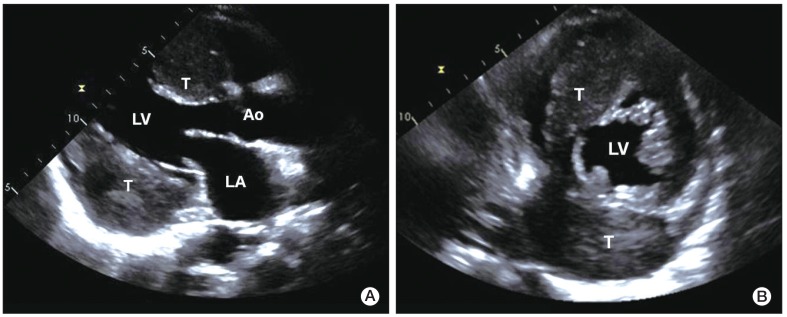
Transthoracic two-dimensional echocardiography. Parasternal long axis view (A) and short axis view (B) showing heterogeneous round-shaped masses attached to the pericardium of the interatrial septal groove and the inferolateral wall of the left ventricle, with myocardial involvement. LA, left atrium; LV, left ventricle; Ao, aorta; T, tumor.
An ultrasonographically-guided needle biopsy was performed on the mass in the tail of the pancreas. The tumor showed a nesting growth pattern with monotonous cells. Most tumor cells had centrally-located nuclei with moderate amounts of amphophilic cytoplasm. Nuclei were generally round to oval in shape, with coarsely clumped chromatin by hematoxylin and eoxin staining (Fig. 4A). Immunohistochemical staining showed that tumor cells were positive for pancytokeratin (1:300, Leica, Newcastle upon Tyne, UK), synaptophysin (1:200, NeoMarkers, Fremont, CA), chromogranin (1:200, Leica) (Fig. 4B), and CD56 (1:150, Leica) (Fig. 4C), but negative for vimentin (solid-pseudopapillary neoplasm marker, 1:200, Dako, Glostrup, Denmark), leukocyte common antigen (hematopoietic neoplasm marker, 1:100, Dako), and CEA (pancreatic ductal adenocarcinoma marker, 1:200, Leica). The proliferation index (Ki-67) and mitosis of the neoplastic cells were approximately 15% and <2/10 high power field, respectively. Accordingly, based on histological and immunohistochemical findings, a diagnosis of grade 2 well-differentiated pNET was made. A percutaneous biopsy of the tumor in the left ventricular apical pericardium was performed using a xiphoid approach; results matched the pathologic findings in the pancreatic mass. Therefore, the patient was diagnosed as having nonfunctioning malignant pNET with multiple metastases, including cardiac metastases.
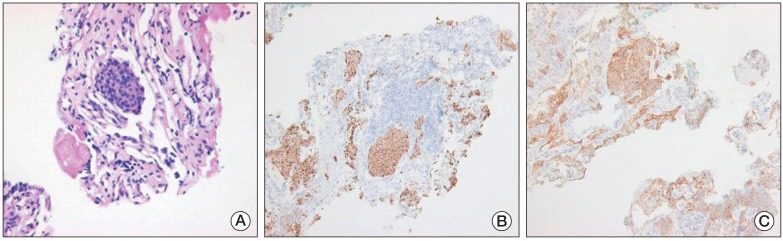
Pathologic features of a needle biopsy of the mass in the pancreatic tail. (A) The tumor showed a nesting growth pattern with monotonous cells. The majority of tumor cells had a centrally located nucleus with moderate amounts of amphophilic cytoplasm. Nuclei were generally round to oval in shape, with coarsely clumped chromatin (H&E staining, ×400). Immunohistochemistry showed that the tumor cells were positive for chromogranin (B) and CD56 (C) (B and C, ×100).
The patient was treated with six courses of etoposide and cisplatin chemotherapy and achieved stable disease status according to the Response Evaluation Criteria In Solid Tumors (RECIST) guidelines. However, the patient's tumorrelated abdominal symptoms worsened. Due the patient's economic circumstances, further treatment with anti-tumor therapy, such as streptozocin or molecular targeting agents, was discontinued, however, the patient continued to receive the best supportive care, including pain control. At 16 months follow-up from the date of diagnosis (in April 2010), the patient underwent percutaneous transhepatic biliary drainage followed by insertion of a biliary stent for treatment of a common bile duct obstruction caused by the presence of progressive pancreatic masses. At 33 months from the date of initial diagnosis (September 2011), the patient developed chest discomfort and exertional dyspnea, and his abdominal and left hip pain, as well as his anorexia, worsened. The patient's general condition deteriorated to Eastern Cooperative Oncology Group performance status 3.
Discussion
In principle, all malignant tumors can metastasize to the heart. In fact, cardiac metastatic disease is more common than primary heart tumors [6], and its incidence has increased during recent decades due to the prolonged survival of patients with cancer and the increased prevalence of the disease in the general population [7-9]. Neoplasms that most commonly metastasize to the heart include malignant melanoma, lymphoma, and leukemia; however, overall numbers are greater for breast and lung cancer, reflecting their higher incidence. In pNET, the liver is the most commonly invaded organ by direct extension or metastasis, followed by regional lymph nodes, and bone [4], whereas cardiac metastasis from pNET has not been previously reported.
The heart is rarely the first site for metastases, and they are usually only found later when diffuse involvement of other organs has already occurred. Tumor cells can metastasize to the heart and pericardium by one of four different pathways: lymphatic, hematogenous, direct extension, or transvenous extension via the superior or inferior vena cava [8,9]. Lymphatic spread often gives rise to pericardial metastases, whereas hematogenous spread has a propensity to migrate to myocardium. Tumors that are in close proximity to the heart, such as bronchial, breast, and esophageal tumors, usually spread by direct extension and give rise to pericardial disease. The most common site of cardiac involvement is the pericardium, with frequencies ranging from 62% to 81%, whereas myocardial or endocardial involvement is rare. In the case described here, metastatic cardiac involvement of pNET occurred in a disseminated setting with regional lymph node and bone metastases, and, via lymphatic spread, multiple pericardial metastases with myocardial involvement.
In such cases, cardiac metastases are usually asymptomatic because the masses are small and do not affect cardiac function [8,9]. When present, clinical signs and symptoms of cardiac metastases are extremely variable, depending on the site and intensity of involvement. Symptoms include dyspnea or tachypnea, and clinical signs include systolic heart murmur, peripheral edema, and pleural or pericardial effusion. Hypotension, peripheral cyanosis, pulsus paradoxus, and venous congestion are also hallmarks of clinical diagnosis. Cardiac metastases can cause disturbances of atrial and ventricular heart rhythm, as well as conduction defects. Pericardial involvement can lead to development of pericarditis with pericardial effusion, which may compromise hemodynamics. Angina pectoris, myocardial infarction, syncope, and sudden death have been reported. Although cardiac metastases cause mild symptoms, more extensive spread of tumors to the pericardium or to other cardiac sites may lead to production of dramatic clinical patterns, resulting in occurrence of medical emergencies and sudden death, unlike other metastatic sites. In the case described here, the diagnosis of cardiac metastases resulted from an incidental finding during a metastatic work-up; there were no early cardiac symptoms. Approximately three years from the initial diagnosis, the patient's cardiac symptoms were chest discomfort and dyspnea on exertion.
No standard treatment modality has been established for cardiac metastases, and, because most patients have disseminated disease, the treatment is generally primary tumor systemic therapy. Therefore, treatment of cardiac metastases is mainly confined to palliation of cardiac symptoms, which may improve the quality of life of affected patients. Surgical resection is only indicated in exceptional cases of solitary intracavitary heart metastases that obliterate cardiac chambers or cause valvular obstruction. Malignant pericardial effusion, especially cardiac tamponade, requires percutaneous pericardiocentesis within a short period of time. Radiotherapy for treatment of cardiac metastases should be used with caution as it may lead to fibrosis of the lung or myocardium, and may be associated with disturbance of the conduction system and pericarditis. The chemotherapeutic agents doxorubicin, daunorubicin, and high-dose cyclophosphamide have cardiotoxic side effects, which may induce recalcitrant myocardial failure.
Systemic chemotherapy for advanced pNETs has been studied in many clinical trials over the past three decades; however, despite a multitude of publications, debate over its efficacy continues. Streptozocin alone or in combination with doxorubicin remains the only chemotherapeutic agent approved for treatment of advanced pNETs; however, it is associated with considerable adverse events [10,11]. Recent large prospective trials have shown that the molecular targeting agent, sunitinib, a multi-target receptor tyrosine kinase inhibitor, and the mammalian target of rapamycin inhibitor, everolimus, has promising antitumor activity [12, 13]. Due to his economic circumstances, our patient was not treated with streptozocin or a molecular targeting agent, and instead received etoposide and cisplatin but failed to respond. Nevertheless, he has been relatively well for the last 33 months; patients with pNETs can live with metastatic disease for several years.
This report describes a case of nonfunctioning pNET with multiple metastases to previously undescribed cardiac sites. Although rare, pNET has the potential to metastasize to the heart or pericardium, and, for patients with pNETs who present with new cardiac symptoms or signs, whether based on history or physical examination, the clinician should be alert to the possibility of cardiac metastases.
Notes
Conflict of interest relevant to this article was not reported.