Longitudinal Changes in Smoking Behaviors and Cancer-Related Mortality Risk in Middle-Aged Korean Women
Article information
Abstract
Purpose
This study investigated association between smoking habit change and cancer-related mortality risk in Korean women.
Materials and Methods
Study population were women aged ≥ 40 years who underwent two biennial cancer screenings during 2009-2012 and were followed up until 2020. Participants were grouped into sustained nonsmokers, sustained quitters, new quitters, relapsers/smoking initiators, and sustained smokers. Outcomes included all-cause and cancer-related deaths. Cox regression and competing risk analysis was used to assess association between smoking habit change and mortality risk.
Results
Of 2,892,590 women, 54,443 death cases were recorded (median follow-up of 9.0 years). Compared with sustained nonsmokers, mortality risk from all causes and cancer-related causes increased in all other smoking groups. Cancer-related risk increased 1.22-fold among sustained quitters (95% confidence interval [CI], 1.10 to 1.36), 1.56-fold (95% CI, 1.40 to 1.75) in new quitters, 1.40-fold (95% CI, 1.21 to 1.62) in relapsers/smoking initiators, and 1.61-fold (95% CI, 1.46 to 1.78) in sustained smokers compared with sustained nonsmokers. Women who were sustained smokers with higher smoking intensity had a higher mortality risk in terms of hazard ratios compared to nonsmokers (< 5 pack-years 2.12-fold, 5-10 pack-years 2.15-fold, and > 10 pack-years 2.27-fold).
Conclusion
Quitting smoking earlier is critical for preventing death from all causes and cancer among female smokers.
Introduction
More than one-fifth of total cancer deaths are attributable to smoking, making it a strong risk factor and the most significant preventable cause of cancer [1,2]. Tobacco use increases the risk of several types of cancer [3] and smoking cessation is associated with a delay and reduction in cancer-related deaths, particularly lung cancer [4-7].
Despite the abundant evidence on cancer mortality risk in smokers, most existing studies measured the changes in smoking behavior in former and past smokers using only one baseline survey and did not consider longitudinal changes in smoking behaviors during the study period. In addition, epidemiological evidence on the association between smoking and mortality from cancer has remained limited or outdated [8,9] and is mainly found in Western populations [8,9].
In Asian countries, where smoking prevalence in women is much lower than in men [10], smoking or changes in smoking behaviors in women have been given less attention. Meanwhile, the smoking rate among Korean women has gradually increased in recent years [11]; however, epidemiological studies on the effect of smoking on Korean women have remained limited. Therefore, this cohort study was conducted to provide an updated evaluation of the association between longitudinal changes in smoking habits and mortality risk, especially in Asian women. This study assessed the association between changes in smoking habits and the risk of death from all and cancer-related causes. We further assessed how mortality risk differed according to smoking intensity among current smokers.
Materials and Methods
1. Study participants
We used data from the National Health Insurance Service–National Health Information Database (NHIS-NHID) [12]. In Korea, the NHIS provides biennial health-screening examinations for all Korean individuals to assess the risk of chronic diseases. All inpatient and outpatient visits, procedures, and prescriptions were recorded in the database.
The initial study cohort included women aged ≥ 40 years who underwent breast cancer screening between 2009-2010 (n=5,105,129) and 2011-2012 (n=5,641,764), considering the biennial interval of breast cancer screening in Korea. Participants were followed up until the date of death or December 31, 2020, whichever came first. Only participants who underwent screening in both periods, with information available on smoking status, were initially considered (n=3,299,776) (Fig. 1). Among them, participants who were aged < 40 or > 75 years, and who had missing information on smoking status, missing information on date of death, or self-reported history of cancer, stroke, or ischemic heart disease before the date of screening were excluded. We excluded participants aged < 40 or > 75 years on the basis of the recommendations for target ages for breast cancer screening [13,14]. After exclusion, 2,892,590 participants were included in the analysis. More details on the study population was described elsewhere [15]. The NHIS database was constructed after anonymization for individual identification.
2. Measures of change in smoking status
Participants reported their smoking status using a self-reported questionnaire during screening. Using the responses from the question, ‘Have you ever smoked 100 cigarettes or more in the course of your lifetime?,’ smoking habits were categorized into three groups: never, former, and current smokers. According to changes in smoking status from the screening in 2009-2010 to the screening in 2011-2012, participants were grouped into the following categories: sustained nonsmokers (those reported as never smokers in both measures), sustained quitters (those reported as former smokers in both measures), new quitters (those reported as current smokers in the first measure and former smokers in the second measure), relapsers (former smokers in the first measure and current smokers in the second measure), smoking initiators (those who were never smokers in the first measure and current smokers in the second measure), and sustained smokers (those who were current smokers in both measures). Owing to the relatively small number of relapsers and smoking initiators, these two groups were combined into one group during the analysis. Additionally, we excluded participants whose smoking status has switched from current smokers to never smokers (n=21,304, 0.6%) or from former smokers to never smokers (n=8,767, 0.3%), which were considered as reporting errors. Owing to the small sample size, we also excluded participants whose status changed from never smokers to former smokers (n=13,098, 0.4%). Smoking intensity was assessed using smoking-pack-years, with one pack-year defined as smoking 20 cigarettes per day for 1 year.
3. Measures of outcomes
The outcomes of interest were death from all causes, cancer-related causes, smoking-related cancers, and lung cancers. By linking our database with the Death Registration Database provided by the Korea National Statistics, we identified the date of death and death causes using the 10th International Classification of Diseases. Smoking-related cancers include those of the pharynx, esophagus, stomach, colon, rectum, liver, pancreas, larynx, lung, bladder, and kidney [16].
4. Measures of other covariates
Data on health behaviors, family history of cancer, and reproductive factors were collected using self-administered standardized questionnaires during health screening between 2009-2010. We considered the following covariates in our analysis: participants’ age at screening (continuous variable) [17,18], body mass index (continuous variable) [18,19], Charlson comorbidity index (CCI) (continuous and categorical variable) [20], number of parities (none, one, two, or more, missing) [18], age at menarche (< 15 years, 15-16 years, > 16 years, missing) [18], menopausal status (premenopausal, postmenopausal, missing) [18], breastfeeding history (never, ever, missing) [18], oral contraceptive use (never, ever, missing) [18], family history of cancer in first-degree relatives (yes, no, missing) [18], alcohol consumption (yes, no, missing) [18], weekly physical activity (yes, no, missing) [18], hormone replacement therapy among postmenopausal women (never, ever, missing) [18], and smoking amount measured using pack-years. The CCI was calculated based on 17 conditions classified using International Classification of Diseases, 10th revision codes within a 1-year look-back period prior to the screening date [20,21]. The sum of the weights score is the CCI and was further divided into 0, 1, 2, and 3 points or more [21].
5. Statistical analysis
Descriptive statistics of the baseline characteristics of the participants according to smoking pattern are presented. To quantify the association between changes in smoking habits and all-cause mortality, we performed Cox proportional hazards regression analysis to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs). To determine the association between changes in smoking habits and cancer-related mortality, we used competing risk analysis, considering deaths from other causes as competing events. The time-to-event period was counted in days from the date of the second screening to the date of the event or the end of the study period. The cancer risk was calculated after adjusting for other covariates. Missing covariate values are treated as dummy variables (separate categories).
We assessed the mortality risk in the different smoking pattern groups compared with the sustained nonsmoker group. The smoking pattern variable, with five values corresponding to the five smoking groups, was included in one regression model, and the sustained nonsmoker group was used as the reference. We further assessed the mortality risk according to smoking intensity among sustained smokers compared to sustained nonsmokers. Smoking intensity was measured by smoking pack-years and categorized as < 5 pack-years, 5-10 pack-years, and > 10 pack-years. Statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC).
Results
A total of 2,892,590 women were followed up during an average of 8.9 years (standard deviation, 0.8 years; median, 9.0 years; interquartile range, 8.4 to 9.4 years), with 54,433 death cases detected (Fig. 1). Of 2,892,590 women, 49,993 (1.7%) were sustained smokers, 29,269 (1.0%) were new quitters, and 45,850 (1.6%) were sustained quitters. The mean age was lowest in the sustained smoker group (53.3±8.2) and highest in the sustained nonsmoker group (56.2±8.9) (Table 1).
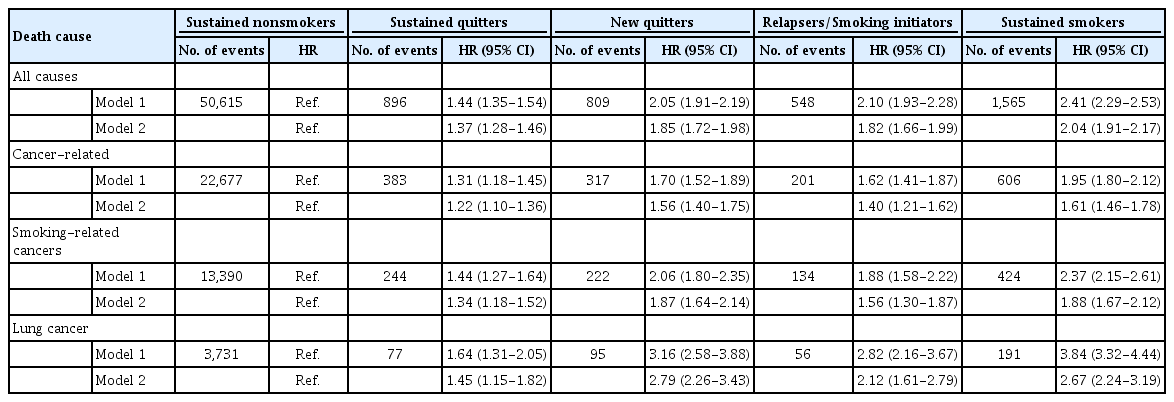
The risk of mortality in the other smoking groups relative to that in sustained nonsmokers is presented in Table 2. In the first model with only adjustment for age, the mortality risk increased in all smoking groups compared to sustained nonsmoker groups, with the HR of 1.44-fold (95% CI, 1.35 to 1.54) in sustained quitters, 2.05-fold (95% CI, 1.91 to 2.19) in new quitters, 2.10-fold (95% CI, 1.93 to 2.28) in relapsers/smoking imitators, and 2.41-fold (95% CI, 2.29 to 2.53) in sustained smokers. After additionally adjusting for other covariates, such as smoking pack-years and comorbidity index, the HR values decreased but remained statistically significant. Compared to sustained nonsmokers, the risk of mortality from all causes increased in all other smoking groups: 1.37-fold (95% CI, 1.28 to 1.46) in sustained quitters, 1.85-fold (95% CI, 1.72 to 1.98) in new quitters, 1.82 (95% CI, 1.66 to 1.99) in relapsers/smoking imitators, and 2.04-fold (95% CI, 1.91 to 2.17) in sustained smokers. In terms of cancer-related deaths, the highest risk was also observed in the sustained smokers group relative to nonsmokers (adjusted hazard artio [aHR], 1.61; 95% CI, 1.46 to 1.78), followed by new quitters (aHR, 1.56; 95% CI, 1.40 to 1.75), relapsers/smoking initiators (aHR, 1.40; 95% CI, 1.21 to 1.62), and sustained quitters (aHR, 1.22; 95% CI, 1.10 to 1.36). Compared to the overall cancer-related risk, a stronger association was found with the risk of death and smoking-related cancers, especially lung cancer. Women in the sustained smoking group had a 1.88-fold (95% CI, 1.67 to 2.12) and 2.67-fold (95% CI, 2.24 to 3.19) increased risk of smoking-related cancers and lung cancer deaths, respectively. For women that were new quitters, the HR of smoking-related cancer mortality and lung cancer mortality were 1.87 (95% CI, 1.64 to 2.14) and 2.79 (95% CI, 2.26 to 3.43), and the corresponding values of relapsers/smoking initiators were 1.56 (95% CI, 1.30 to 1.87) and 2.12 (95% CI, 1.61 to 2.79).
Among sustained smokers, the risk of mortality increased, corresponding to a higher smoking intensity (Table 3). Compared to the nonsmoker group, the overall mortality risk in sustained smokers with < 5 pack-years, 5 to 10 pack-years, and > 10-pack-years was 2.12-fold (95% CI, 1.90 to 2.35), 2.15-fold (95% CI, 1.93 to 2.40), and 2.27-fold (95% CI, 2.12 to 2.43), respectively. A similar pattern was observed in the risk of cancer-related, smoking-related, and lung cancer deaths. The risk of cancer-related deaths increased 1.76-fold (95% CI, 1.41 to 2.19), 2.19-fold (95% CI, 1.79 to 2.69), and 2.49-fold (95% CI, 2.02 to 2.83), respectively. Sustained smokers with > 10 pack-years had 2.49-fold (95% CI, 2.20 to 2.83) and 4.36-fold (95% CI, 3.63 to 5.23) increased risk of death from smoking-related cancers and lung cancer, respectively.
Discussion
The findings from this study add to the growing evidence that mortality among sustained smokers is twice as high as that among women who never smoked. The increased risks of mortality due to cancer, smoking-related cancer, and lung cancer ranged from 1.6-fold to 2.7-fold. We additionally found that mortality risk differed according to changes in smoking habits, with the lowest risk in sustained quitters and highest risk in sustained smokers. Women who had smoked for 10 or more pack-years might have twofold increased risk of dying from smoking-related cancers and 4 times increased risk of dying from lung cancer.
Although the smoking rate is relatively lower among Asian women compared to that in the female Western population [22], the smoking rate among Korean women increased from 5.3% to 7.5% from 2007 to 2018 [11]. Due to the large difference in smoking rates among Korean women (7.5% in 2018) compared to Korean men (36.7% in 2018), epidemiological studies on the effect of smoking among Korean women have remained limited. Prior to this study, there is broad agreement that cigarette smoking increased the mortality risk by 2 to 3 times in female smokers compared to women who never smoked [8,9,23,24]. Although evidence on smoking and mortality risk in female smokers has been comprehensively reported, these findings were mostly in Western populations [8,9,23] who might have different smoking prevalence and smoking patterns than those of Asian female smokers. In line with our findings, findings from the Nurses’ Health Study cohort report that current smokers had a 2.8-fold (95% CI, 2.68 to 2.95) increased risk of total mortality [8]. Another pooled analysis of five US cohort studies also reported an overall increase of 2.8-fold (95% CI, 2.7 to 2.9) risk of all-cause mortality in female current smokers [23]. It should be noted that while findings on increased mortality risk associated with lung cancer and smoking-related factors in our results and previous studies are consistent, the magnitude of association was relatively lower than that of our findings. The risk of death from lung cancer increased 12-fold in the Nurses’ Health Study cohort [8] and 23-fold in a pooled analysis study [23]. The lower smoking intensity in Asian women [10,25], including Koreans, and the shorter follow-up time in our study might explain the discrepancy in lung cancer-related risk.
Our findings suggest a dose-response relationship between smoking intensity and risk of mortality among sustained smokers, with a higher HR in those with longer smoking pack-years. The risk of cancer-related deaths increased from 1.6-fold in those with < 5 pack-years to 2-fold in those with more than ten pack-years, and the risk of lung cancer deaths increased from 2-fold to > 4-fold, respectively. Consistent with our findings, previous studies also found a dose-response relationship between the intensity of smoking, measured as either the number of smoked cigarettes [8,26] or smoking pack-years [27], and mortality risk in female smokers. A pooled analysis of Asian female smokers reported a higher risk corresponding to higher smoking pack-years, with a 1.6-fold increase in all-cause mortality in those with < 10 pack-years to a 2-fold increase in women with ≥ 20 packyears [27]. Although smoking intensity is lower in Asian women [10,25] recent studies also reported that smokingrelated cancer mortality risk increased even in low-intensity and lifelong non-daily smokers compared to never smokers [28-30].
In this study, the risk of death from lung cancer was found to be highest in the new quitter group followed by the sustained smoker group. In terms of all-cause and cancer-related deaths, the HR of sustained smokers was higher than that of sustained nonsmokers, followed by new quitters. The unique findings regarding lung cancer might be due to the changed smoking status as an intermediate variable. Development of fatal diseases or deteriorating health condition that occur during the period between the two measures may affect smoking habits and subsequently act as an intermediate variable between smoking habit change and increased mortality risk. A woman might receive a lung cancer diagnosis and then decide to quit smoking, and this diagnosis may increase her risk of death due to lung cancer. In our analysis, the mean CCI in the new quitter group was higher than that of sustained smokers, sustained quitters, and nonsmokers (0.480 vs. 0.479, 0.443, and 0.442, respectively; data not shown). In our previous study [15], we found that changes in smoking behavior were associated with an increased incidence of cancer (all types), smoking-related cancers, and lung cancer. The risk was the highest in sustained smokers, followed by new quitters, relapsers/smoking inhibitors, and sustained quitters. Consequently, this increased cancer risk explains the increased mortality risk observed in the current study. A similar pattern was observed in the association between changes in smoking status and cancer-related mortality risk. The mortality risk from cancer-related, smoking-related, and lung cancers was also the highest in women who reported as sustained smokers or new quitters. The combined evidence from our previous and current studies suggests that changes in smoking status are associated not only with cancer risk but also with all-cause and cancer-related mortality risk in female smokers.
This study has several limitations. First, information on smoking was collected from a self-reported questionnaire, which may not accurately reflect the participants’ smoking habits, especially due to the social stigma regarding smoking among Asian women. A study conducted among Korean women recorded a self-reported smoking rate of 7.4%; however, after checking for urinary cotinine levels, the smoking rate was 11.8% [31]. Thus, we can assume that the current smoking rate in Korean women might have been underestimated by 30%-40%, which could have raised potential biases in our study. The underestimation of current smoking status might cause a non-differential bias, because the degree of misclassification of smoking status would not be systematically different between death and survival. A non-differential bias could lead to an observed association with the null [32]. Thus, the results of this study would underestimate the increased mortality risk associated with changes in smoking habits. Meanwhile, it should be noted that in this study, smoking habits were assessed twice within a two-year period in hospital settings, which might help increase the accuracy of smoking status. Second, we did not have information on the age and duration of quitting among smoking quitters; therefore, we were not able to perform an analysis on the benefit of smoking cessation in reducing mortality risk. Third, the median follow-up time was 9 years, which was relatively short and insufficient to assess the effect of smoking on cancer mortality risk, especially for slow-growing diseases, such as cancer. Finally, our database might have been subjected to selection bias because women who regularly undergo cancer screening may be a particularly health-conscious segment of the population.
The strengths of this study include a very large, female, population-based, longitudinal assessment of smoking status and accurate assessment of death cases. In conclusion, this cohort study showed that smoking status is associated with all-cause and cancer-related mortality risk in female smokers, with the highest risk in sustained smokers, new quitters, relapsers/smoking initiators, and sustained quitters compared with those who never smoked. In addition, a higher smoking intensity was associated with a higher mortality risk by up to four times in lung cancer deaths among women with 10 pack-years or more.
Notes
Ethical Statement
This study was approved by the Institutional Review Board of the Hanyang University College of Medicine (approval No. HYUIRB-202106-003-1). The NHIS database approved the National Health Insurance Sharing Service system. In this study, all screened populations agreed to transfer their screening results to the NHIS-NHID; therefore, the requirement for informed consent was waived.
Author Contributions
Conceived and designed the analysis: Tran TXM, Kim S (Soyeoun Kim), Kim S (Seonju Kim), Park B.
Collected the data: Tran TXM, Kim S (Soyeoun Kim), Kim S (Seonju Kim).
Contributed data or analysis tools: Tran TXM, Kim S (Soyeoun Kim), Park B.
Performed the analysis: Tran TXM.
Wrote the paper: Tran TXM, Park B.
Supervision: Park B.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
This work was supported by a National Research Foundation of Korea grant funded by the Korean government (MSIT) (grant no. 2021R1A2C1011958). This work was partly supported by Institute of Information & Communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) (No.2020-0-01373, Artificial Intelligence Graduate School Program (Hanyang University)) and the research fund of Hanyang University (HY-202300000000174).