Long-term Complete Remission of Decitabine-Primed Tandem CD19/CD22 CAR-T Therapy with PD-1 and BTK Inhibitors Maintenance in a Refractory Primary Central Nervous System Lymphoma Patient
Article information
Abstract
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive non-Hodgkin’s lymphoma that affects the brain, eyes, cerebrospinal fluid, or spinal cord without systemic involvement. The outcome of patients with PCNSL is worse compared to patients with systemic diffuse large B-cell lymphoma. Given potential mortality associated with severe immune effector cell-associated neurotoxicity syndrome (ICANS), patients with PCNSL have been excluded from most clinical trials involving chimeric antigen receptor T-cell (CAR-T) therapy initially. Here, we report for the first time to apply decitabine-primed tandem CD19/CD22 dual-targeted CAR-T therapy with programmed cell death-1 (PD-1) and Bruton’s tyrosine kinase (BTK) inhibitors maintenance in one patient with multiline-resistant refractory PCNSL and the patient has maintained complete remission (CR) for a 35-month follow-up period. This case represents the first successful treatment of multiline resistant refractory PCNSL with long-term CR and without inducing ICANS under tandem CD19/CD22 bispecific CAR-T therapy followed by maintenance therapy with PD-1 and BTK inhibitors. This study shows tremendous potential in the treatment of PCNSL and offers a look toward ongoing clinical studies.
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare and aggressive type of extranodal lymphoma with majority cases limited involving the central nervous system (CNS) at onset, with diffuse large B-cell lymphoma (DLBCL) being the most common histologic subtype. Since PCNSL is a malignancy arising in an immune-privileged site, suboptimal delivery of systemic agents into tumor tissues results in poorer outcomes in PCNSL than in systemic DLBCL [1]. The current therapeutic strategies for PCNSL include high-dose methotrexate (HD-MTX)–based chemotherapy, whole-brain radiation therapy (WBRT), and autologous hematopoietic stem cell transplantation. Despite high rates of response, relapse is common. Almost half of the PCNSL patients will experience relapse or refractory to treatment. Data from the French LOC network shows that about 29% of PCNSL patients fail in first-line treatment. About 10%–15% of patients are refractory to standard treatment and prognosis tends to be much worse in refractory patients, with a median overall survival of only 2 months without treatment intervention [2]. Novel therapeutic strategies to improve the prognosis of this continuously increasing patient population are urgently needed.
Novel therapeutic approaches using targeted agents may be particularly attractive for patients who are resistant to HD-MTX–based regimens. Targeted drugs including Bruton’s tyrosine kinase (BTK) inhibitor, immune modulatory small molecules, and checkpoint inhibitor programmed cell death-1 (PD-1) inhibitor are promising in PCNSL according to the literature reported. Though new targeted therapies have shown high response rates, the responses are not long-lasting and are prone to relapse [2]. Up to now, chimeric antigen receptor T-cell (CAR-T) therapy has achieved excellent feedback on multiple types of B-cell malignancies. Due to potential mortality associated with severe immune effector cell-associated neurotoxicity syndrome (ICANS), patients with PCNSL have been excluded from clinical trials involving CAR-T therapy initially [3]. Recently, it has been reported that CAR-T cells can go through the blood-brain barrier and control CAR-T cell-related encephalopathy syndrome [1]. Also some successful cases about CAR-T therapy in PCNSL have been reported though limited to case reports or phases I/II clinical trials [4,5]. Still these studies suggest that CAR-T therapy is not an absolute contraindication for PCNSL. Herein, we report for the first-time successful treatment in a multiline-resistant refractory PCNSL patient by applying tandem CD19/CD22 CAR-T therapy.
Case Report
A 49-year-old male presented with a one-week history of left-sided limb weakness and slurred diction was administered. Cranial magnetic resonance imaging (MRI) showed occupancy in the brainstem, right frontoparietal temporal lobe, and basal ganglia area (Fig. 1A and E). Positron emission tomography–computed tomography scanning excluded the involvement of other body sites. Intracranial stereotactic biopsy confirmed the diagnosis of PCNSL (non-germinal center B-cell subtype) in 2019.

Clinical efficacy after chimeric antigen receptor T-cell (CAR-T) therapy in a multiline-resistant refractory primary central nervous system lymphoma patient. Representative magnetic resonance imaging of untreated, before CAR-T infusion, 1 month after CAR-T infusion and 27 months after CAR-T infusion were shown. (A, E) Occupancies in the brainstem, right frontoparietal temporal lobe, and basal ganglia area were observed at diagnosis. (B, F) The previous lesions decreased dramatically, but a new left-sided lesion appeared before CAR-T therapy. (C, G) no brain lesions were shown 1 month after CAR-T therapy. (D, H) The patient remained in complete remission until now. The red arrow pointed to the location of the tumor.
The patient was first treated with 375 mg/m2 rituximab combined with 3.5 g/m2 MTX in August 2019. After the treatment, the patient presented with deteriorated weakness in both lower limbs. Further cranial MRI showed new lesions at bilateral corticospinal tracts suggesting disease of progression (PD). The treatment was then switched to high-dose of cytarabine (2 g/m2 q12h d1-d2) in September 2019, but the results were still frustrating. Only after 13 days, the patient got syncope and the cranial contrast computed tomography showed multiple enlarged occupancies in the right frontotemporoparietal lobe. Then the patient was subsequently treated with targeted drugs including lenalidomide and ibrutinib. Unfortunately, the result was still unsatisfactory. In October 2019, a whole-brain radiation treatment of 45 Gy in 10 fractions was performed followed by injection of three doses PD-1 inhibitor camrelizumab. However, the patient got no syndrome improved and the MRI showed new tumor lesion (Fig. 1B and F).
In cases of ineffective treatment approaches, we came up with the idea of CAR-T therapy. Based on the immunohistochemical staining for markers shows strong positive for CD19 and CD22 (Fig. 2A and B), the patient was exposed to leukapheresis for the isolation of peripheral blood mononuclear cells to manufacture the 2nd generation 41BB-CD3zeta tandem CD19/22 CAR-T cells. The tandem CD19/CD22 dual-targeted CAR-T cells were prepared by Shanghai Unicar-Therapy Bio-medicine Technology Co. Ltd. as described previously [6]. In March 2020, the patient was pretreated with DFC regimen (decitabine a total dose of 100 mg/m2 equally intravenously administrated for 3 consecutive days, cyclophosphamide 300 mg/m2 d1-d3, fludarabine 30 mg/m2 d1-d3) for lymphocyte depletion, and then a total dose of 1×107/kg CAR-T cells bispecific for CD19/22 were infused at a dose escalation of 10%, 30%, and 60%. Febrile neutropenia (fever peak 38.3°C) and thrombocytopenia were observed and recovered within one month after infusion (Fig. 3A). A Series of serum tests showed that except rapid increase of interleukin 6, C-reactive protein and ferritin, no other adverse events were observed (Fig. 3B–D). The patient presented with grade 2 cytokine releasing syndrome (CRS) and no ICANS.

Immunohistochemical staining for markers shows strong positive for CD19 (A) and CD22 (B), weak positive for programmed cell death-1 (PD-1) (C).
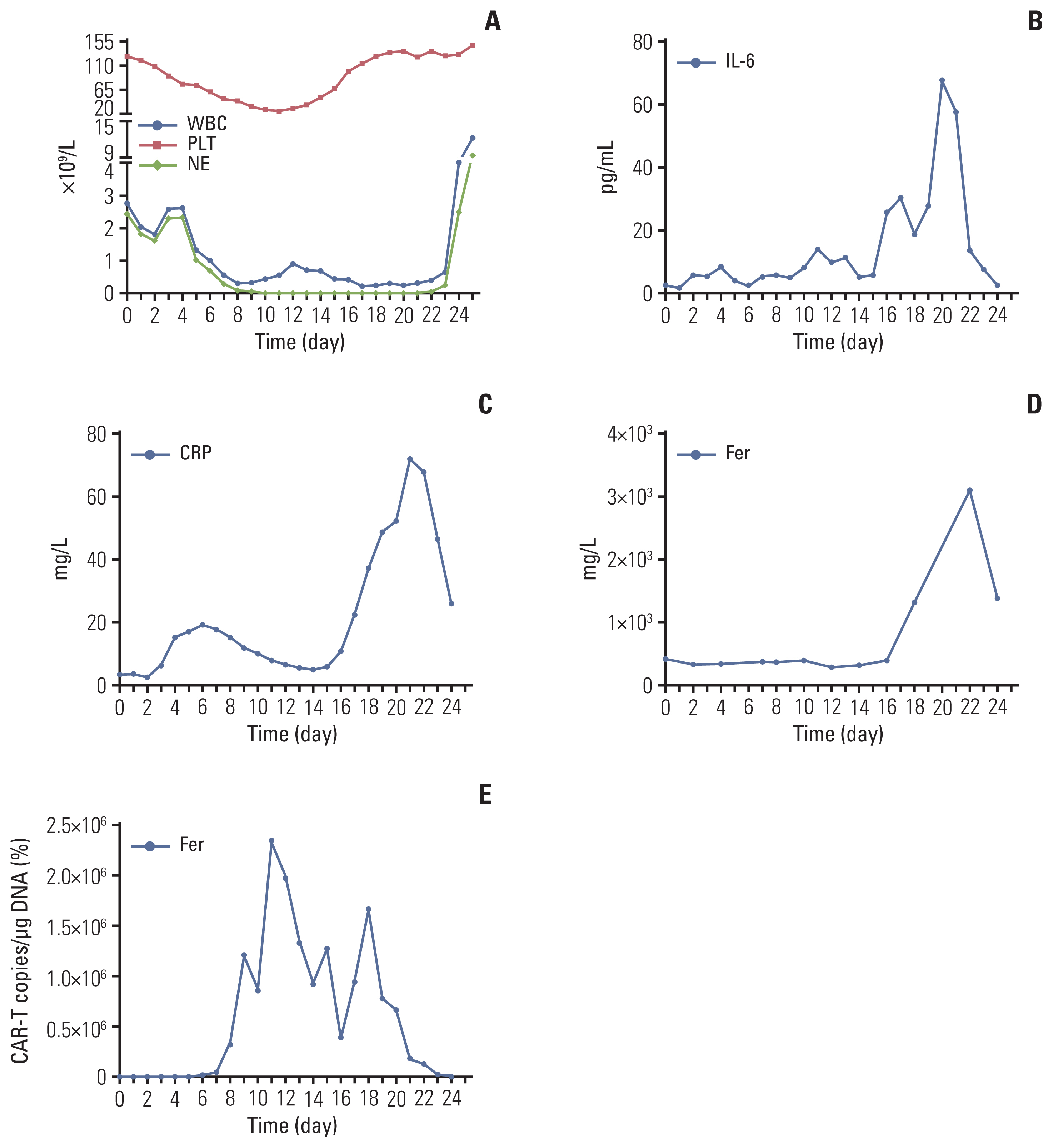
Hematological toxicities, cytokines, and CAR-T copies in a multiline-resistant refractory PCNSL patient. (A) Changes of WBC, NEU, and PLT levels after CAR-T therapy. (B–D) Changes of IL-6, CRP, and Fer levels after CAR-T therapy. (E) Changes of CAR-T copies at the indicated time points after CAR-T therapy. CAR-T, chimeric antigen receptor T-cell; CRP, C-reactive protein; IL-6, interferon 6; NEU, neutrophil; PCNSL, primary central nervous system lymphoma; PLT, platelet; WBC, white blood cell.
After CAR-T infusion, CAR-T copies in peripheral blood at the indicated time points were measured using real-time quantitative polymerase chain reaction and immunofluorescence. Absolute CAR-T copies in peripheral blood were maintained at 9.87×102/μg DNA-2.34×106/μg DNA within one month after CAR-T infusion (Fig. 3E). Further, the patient’s original neurological symptoms gradually disappeared and his physical condition was significantly improved. One month after CAR-T infusion, the patient was exposed to cranial MRI which showed that the patient got complete remission (CR) (Fig. 1C and G). To prevent future relapse after CAR-T therapy, maintenance therapy was supportive. Based on positive PD-1 expression in the tumor tissue (Fig. 2C), PD-1 inhibitor camrelizumab 200 mg was given every three weeks with oral ibrutinib 560 mg daily as maintenance therapy for 1 year since May 2020. Up to now, the patient was still alive and maintained CR (Fig. 1D and H) with a follow-up of up to 35 months. The treatment timeline of the case was summarized in Fig. 4.
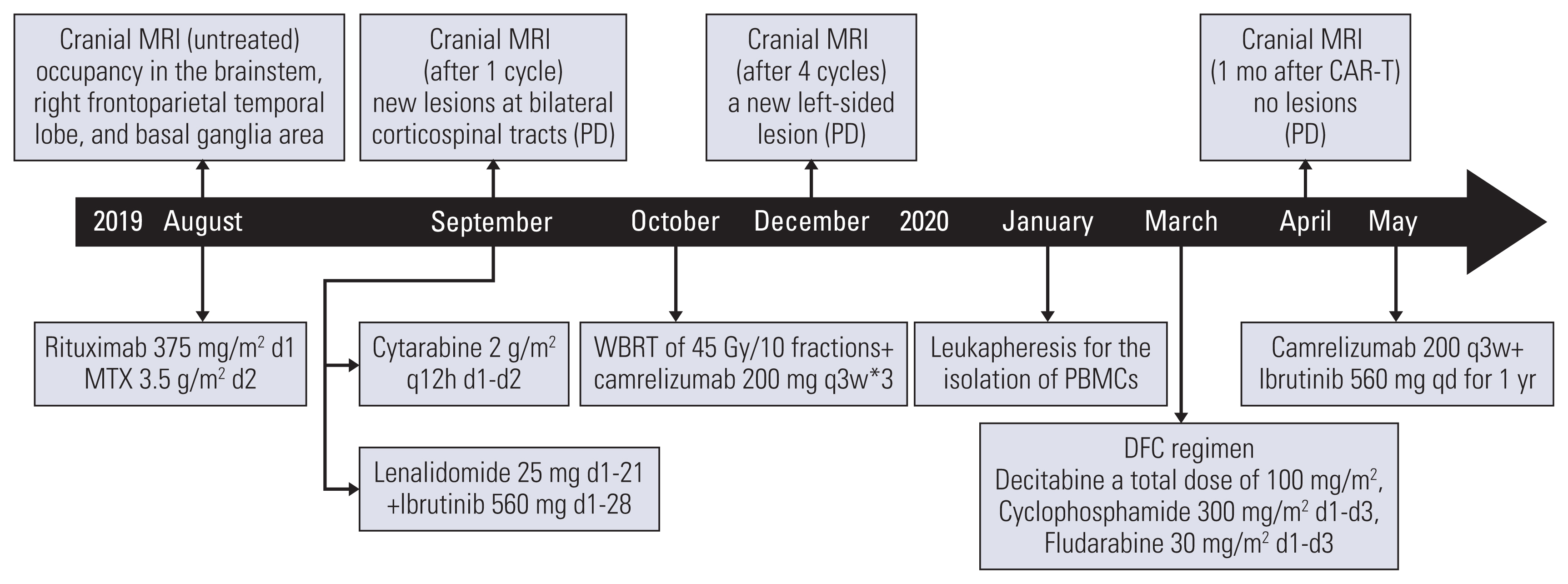
The treatment timeline in a multiline resistant refractory PCNSL patient. CAR-T, chimeric antigen receptor T-cell; MRI, magnetic resonance imaging; MTX, methotrexate; PBMCs, peripheral blood mononuclear cells; PCNSL, primary central nervous system lymphoma; PD, progression of disease; q3w, every 3 weeks; q12h, every 12 hours; WBRT, whole-brain radiation therapy.
Discussion
The efficacy and safety of CAR-T in PCNSL have been reported in a small number of cases in recent years. Tanya Siddiqi et al. [5] reported that three of five PCNSL patients achieved CR after CD19 CAR-T therapy, but all patients developed grade ≥ 1 CRS and ICANS. Frigault et al. [7] reported that 7 of 12 patients demonstrated response and six achieved CR initially with three cases sustained remission with a median follow-up of 12.2 months from infusion, but seven of 12 patients developed grade ≥ 1 CRS and six of 12 patients developed grade ≥ 1 ICANS [7]. Jacobson et al. [8] reported that six of nine patients achieved CR with a median follow-up of 12 months, but seven of nine patients developed grade 1–2 CRS and four of nine patients developed grade ≥ 1 ICANS in the 64th American Society of Hematology annual meeting.
Although new strategies for CD19-directed CAR-T therapy have been developed in recent years, 21%–35% of patients still experience relapse after anti-CD19 CAR-T–induced remission [3]. Simultaneously targeting another tumor antigen to prevent relapse has been proposed [9]. Tu et al. [3] reported a case of a patient with relapsed PCNSL treated with CD19 CAR-T and CD70 CAR-T “Cocktail” therapy who achieved long-term remission without inducing CRS or ICANS. Here, we reported a refractory PCNSL case successfully treated with tandem CD19/CD22 dual-targeted CAR-T therapy. To our knowledge, this was the first case to demonstrate the safety and efficacy of tandem CD19/CD22 dual-targeted CAR-T therapy in a multiline refractory PCNSL patient after a long-term follow-up. Treatment was well tolerated with manageable grade 2 CRS and no ICANS. The long-term remission and absence of severe CRS and ICANS in both cases indicated the potential benefit and safety of dual targets CAR-T therapy applied to PCNSL.
In the literature, Li et al. [10] reported the safety and efficacy of 5 PCNSL patients who received CD19CAR-T and CD22CAR-T “Cocktail” therapy. All toxicities were controllable with all patients experienced CRS and two patients experienced ICANS. All patients demonstrated response with two achieved CR and three partial response initially, but the remission did not last long and four got PD shortly with a median progression-free survival (PFS) of 3 months. Only one case received hematopoietic stem cell transplantation therapy in the 3rd month after CAR-T therapy achieved and maintained CR [10]. Compared to this study, the case in our trial showed more superior PFS. One reason may due to the different design of our CAR-T cells using tandem CD19/CD22 dual targets CAR-T cells other than cocktail with two single target CAR-T cells. One reason may due to the addition of decitabine in the lymphodepletion regimen. Our group previously reported that compared to patients underwent FC lymphodepletion regimen, patients with decitabine-containing DFC regimen present more superior efficacy and PFS after infused with tandem CD19/CD22 dual-targeted CAR-T cells [11]. One reason may due to WBRT before CAR-T therapy. We previously reported that radiation sequential CAR-T therapy in DLBCL patients showed a higher overall response rate and less severe CRS/ICANS. In this case, WBRT may have improved the tumor microenvironment (TME) and reduced the apoptosis and loss of tumor cell surface antigen, thus reducing the occurrence of immune escape during CAR-T treatment and decreasing the probability of disease recurrence [6]. Another reason may due to maintenance therapy with BTK inhibitor and PD-1 inhibitor for 1 year. It has been reported that CD19 CAR-T in combination with BTK inhibitor can further enhance the antitumor effect and lead to a prolonged remission period [12]. Besides, BTK inhibitor can also enhance the antitumor immunity of PD-1 inhibitor [13]. Moreover, programmed death-ligand 1 is overexpressed in TME at the end of CAR-T treatment. This change in TME renders CAR-T therapy ineffective [10]. Application of PD-1 and BTK inhibitors may enhance CAR-T therapeutic effects through improving inhibitory TME in CNS. In this case, though this patient did not benefit from the application of BTK and PD-1 inhibitors initially before CAR-T therapy, he may still benefit from them after CAR-T therapy based on the positive effect of both drugs on CAR-T therapy.
The atypical infection issue due to long-term B-cell lymphodepletion after CAR-T therapy or BTK inhibitor use remained problematic. Up to now, very few studies about BTK/PD-1 inhibitors maintenance after CAR-T therapy were reported in the literature. It was reported that 7% patients experienced grade 3–4 adverse events and 2% of patients developed grade 3–4 pneumonia after combination of CAR-T and BTK inhibitor treatment in a phase 1/2a study [14]. While no infection was observed in patients receiving PD-1 maintenance after CAR-T therapy [15]. Furthermore, Zhang et al. [16] reported one case who received BTK and PD-1 inhibitors maintenance therapy after CD19-directed CAR-T developed no infection but only menorrhagia and anemia. In this case, no adverse events including infection were observed during the 1-year maintenance therapy with BTK and PD-1 inhibitors which was consistent with the previous reports.
In summary, decitabine-primed tandem CD19/CD22 dual-targeted CAR-T therapy with PD-1 and BTK inhibitors maintenance is a safe and promising approach for PCNSL patients from the above case. Of course, there is an inevitable chance of individual case reports. This also needs to be confirmed by more clinical trial data and real-world studies. Besides, our report also suggests CAR-T therapy has unlimited potential for the treatment of involved lymphomas. In the future, CAR-T therapy has the potential to be included in the treatment regimen for patients with PCNSL and to provide benefit to patients with secondary CNS involvement.
Notes
Ethical Statement
The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceived and designed the analysis: Wu D, Jin Z, Qu C.
Collected the data: Zou R, Zhou X, Liu H.
Contributed data or analysis tools: Zhou X, Wang P, Xia F, Kang L, Yu L.
Performed the analysis: Zou R, Liu H.
Wrote the paper: Zou R, Qu C.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.
Acknowledgments
We would like to thank all members of the study team, the patient, and his family. This work was supported by research grants from the National Key R&D Program of China (2016YFC0902800) (to DW), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (to DW), National Natural Science Foundation of China [(81400155) (to CQ and ZJ)], Jiangsu Natural Science Foundation of China (BK20140374) (to CQ and ZJ), Top-notch young health talents, 5th Suzhou health professionals program (GSWS2019035) (to CQ), and National Clinical Research Center for hematologic disease (2021ZKMC01) (to CQ).
