Predictive Value of Interstitial Lung Abnormalities for Postoperative Pulmonary Complications in Elderly Patients with Early-Stage Lung Cancer
Article information
Abstract
Purpose
Identifying pretreatment interstitial lung abnormalities (ILAs) is important because of their predictive value for complications after lung cancer treatment. This study aimed to assess the predictive value of ILAs for postoperative pulmonary complications (PPCs) in elderly patients undergoing curative resection for early-stage non–small cell lung cancer (NSCLC).
Materials and Methods
Elderly patients (age ≥ 70 years) who underwent curative resection for pathologic stage I or II NSCLC with normal preoperative spirometry results (pre-bronchodilator forced expiratory volume in 1 second to forced vital capacity [FVC] ratio > 0.70 and FVC ≥ 80% of the predicted value) between January 2012 and December 2019 were retrospectively identified. Univariable and multivariable regression analyses were performed to assess risk factors for PPCs. The Kaplan-Meier method and log-rank test were used to analyze the relationship between ILAs and postoperative mortality. One-way analysis of variance was performed to assess the correlation between ILAs and hospital stay duration.
Results
A total of 262 patients (median age, 73 years [interquartile range, 71 to 76 years]; 132 male) were evaluated. A multivariable logistic regression model revealed that, among several relevant risk factors, fibrotic ILAs independently predicted both overall PPCs (adjusted odds ratio [OR], 4.84; 95% confidence interval [CI], 1.35 to 17.38; p=0.016) and major PPCs (adjusted OR, 8.72; 95% CI, 1.71 to 44.38; p=0.009). Fibrotic ILAs were significantly associated with higher postoperative mortality and longer hospital stay (F=5.21, p=0.006).
Conclusion
Pretreatment fibrotic ILAs are associated with PPCs, higher postoperative mortality, and longer hospital stay.
Introduction
Lung cancer is a major medical concern in elderly patients and a leading cause of cancer-related mortality [1,2]. The median age at diagnosis of lung cancer is 71 years [3]. Non–small cell lung cancer (NSCLC) accounts for approximately 80% of all lung cancers. Surgery is the standard treatment for early-stage NSCLC and can be safely performed in elderly patients [4]. Recently, a Dutch-Belgian lung cancer screening trial (NELSON [Nederlands–Leuvens Longkanker Screenings Onderzoek]) reported that lung cancer screening can reduce lung cancer mortality [5]. Increased lung cancer screening will lead to increased detection of early-stage lung cancer and, with increasing population aging, increased application of lung cancer surgery in elderly patients.
As elderly patients have many comorbidities and lung cancer surgery can result in more postoperative complications in this group than in younger groups [6], preoperative risk stratification is essential for correcting modifiable risk factors and predicting adverse clinical outcomes, such as postoperative pulmonary complications (PPCs). PPCs are important because they are major factors increasing mortality and morbidity after thoracotomy and are associated with increased costs owing to prolonged hospital stay [7].
Preoperative risk stratification consists of multidisciplinary assessments, including cardiologic evaluation and pulmonary function tests [8]. Preoperative chest computed tomography (CT) is widely used nowadays. In addition to its role in TNM staging, CT can provide useful information, such as the presence of interstitial lung abnormalities (ILAs), in patients without clinically significant abnormalities. ILAs are incidental radiologic findings such as ground-glass attenuation, reticulation, traction bronchiectasis, honeycombing, and nonemphysematous cysts [9]. ILAs have been receiving attention in the era of lung cancer screening. Asymptomatic ILAs were identified to be an independent risk factor for lung cancer in the National Lung Cancer Trial [10]. Pretreatment ILAs are associated with pulmonary morbidity and mortality in patients undergoing lung cancer treatment including chemotherapy [11] and radiation therapy [12]. As ILAs reflect subclinical abnormalities, they can serve as predictors of prognosis after lung cancer surgery in patients with early-stage disease.
This study aimed to assess the predictive value of ILAs for PPCs in elderly patients (age ≥ 70 years) undergoing curative lung resection for early-stage NSCLC.
Materials and Methods
This study was conducted on patients aged ≥ 70 years who underwent curative lung resection at our hospital. The inclusion criteria were as follows: (1) NSCLC of pathologic stage I or II and (2) preserved lung function with normal spirometry results. Clinical and pathologic staging were reevaluated according to the 8th TNM staging system. Normal spirometry results were defined as a pre-bronchodilator forced expiratory volume in 1 second (FEV1) to forced vital capacity (FVC) ratio of > 0.70 and FVC ≥ 80% of the predicted value.
Data on clinical factors were obtained by reviewing the patients’ electronic medical records, including age, sex, body mass index, smoking status, American Society of Anesthesiologists (ASA) physical status classification, comorbidities, serum hemoglobin, serum albumin, percentage predicted FEV1, percentage predicted diffusing capacity of the lung for carbon monoxide (DLCO), and tumor histologic type. Specific information about the operation was also investigated in detail.
All chest CT scans including high-resolution CT images were obtained using the following multidetector CT scanners: LightSpeed 16 (GE Healthcare, Chicago, IL), LightSpeed VCT (GE Healthcare), Somatom Definition Flash (Siemens Healthineers, Erlangen, Germany), and Revolution (GE Healthcare). The slice thickness used at our hospital was 2.0–3.0 mm.
Two thoracic radiologists (W.G.J., 10 years of experience; J.E.L., 7 years of experience) independently reviewed all CT images. We evaluated several imaging features on preoperative chest CT, including ILAs, emphysema, superimposed infection, bronchial wall thickening, and bronchiectasis. ILAs were defined as CT imaging findings that met the following criteria: (1) incidental identification of abnormalities in nondependent lungs (including ground-glass or reticular abnormalities, traction bronchiectasis, honeycombing, and nonemphysematous cysts) and (2) involving at least 5% of a lung zone. When ILAs were observed on CT, they were further classified into the following subcategories: nonsubpleural, subpleural nonfibrotic, and subpleural fibrotic (Fig. 1). Fibrosis was defined as a lung distortion accompanying traction bronchiectasis or honeycombing. This categorization was based on the Fleischner Society classification system [9]. Mild traction bronchiectasis in the subpleural and juxtapleural areas in the absence of architectural distortion was considered as a nonfibrotic ILA [13]. ILAs were further reclassified as nonfibrotic (nonsubpleural, subpleural nonfibrotic) and fibrotic ILAs, according to the presence of fibrosis. The presence of ILAs was determined by consensus of the two designated radiologists. The senior reader (W.G.J.) adjudicated when a consensus was not reached. Thereafter, the subclassification of ILAs into each of the three subcategories was independently performed by two readers who were blinded to the patients’ clinical characteristics. An interobserver reliability test was performed for the subclassification of ILAs.

Subclassification of interstitial lung abnormalities (ILAs) according to the Fleischner Society classification system. (A) Nonsubpleural ILA: high-resolution computed tomography (CT) scan showing ground-glass abnormality with nonsubpleural distribution (white arrows) in both lower lungs. (B) Subpleural nonfibrotic ILA: high-resolution CT scan showing ground-glass abnormality (arrowheads) and mild traction bronchiectasis (white arrow) with subpleural distribution in both lower lungs. There is no evidence of fibrosis. (C) Subpleural fibrotic ILA: high-resolution CT scan showing honeycombing (black arrow), traction bronchiectasis (white arrow), and nonemphysematous cyst (arrowhead) with architectural distortion in both lower lobes.
Most PPCs were defined according to the European Perioperative Clinical Outcome definitions (S1 Table) [14]. Overall PPCs included air leak persisting for > 7 days [15], pneumothorax, pleural effusion, atelectasis, pneumonia, acute respiratory distress syndrome, respiratory failure, and bronchopleural fistula/empyema. Major PPCs included pneumonia, acute respiratory distress syndrome, respiratory failure, and bronchopleural fistula/empyema. When these complications occurred within 60 days after surgery, they were defined as PPCs. The length of hospital stay was also assessed. Rehospitalization for PPCs was included in the length of hospital stay.
All statistical analyses were performed with R statistical software ver. 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS Statistics for Windows ver. 25.0 (IBM Corp., Armonk, NY). Data are presented as number and percentage for categorical variables and median and interquartile range for continuous variables. Univariable and multivariable regression analyses were performed to assess the risk factors for PPCs. The chi-square or Fisher exact tests were used for categorical variables, and an independent t test was used for continuous variables. The interobserver agreement for the subclassification of ILAs was assessed according to the percentage of concordant cases and kappa value of agreement with 95% confidence intervals (CIs). One-way analysis of variance (ANOVA) was performed to assess the correlation between ILA subtypes and length of hospital stay. The Kaplan-Meier method and log-rank test were used to analyze the relationship between ILA subtypes and 30-, 90-, and 180-day mortality. Statistical significance was set at p < 0.05.
Results
Of 712 patients who underwent lung cancer surgery between January 2012 and December 2019, 418 patients were excluded because they had abnormal spirometry results or pathologic stage III or IV lung cancer. We also excluded patients with a history of previous lung cancer or esophageal cancer (n=19), pathologically proven interstitial lung disease (ILD) (n=6), and postoperative non-PPC (chylothorax, 3; bronchial kinking, 1; pericarditis, 1; postoperative bleeding, 1; and cerebral infarction, 1). The final study population comprised 262 patients (Fig. 2).
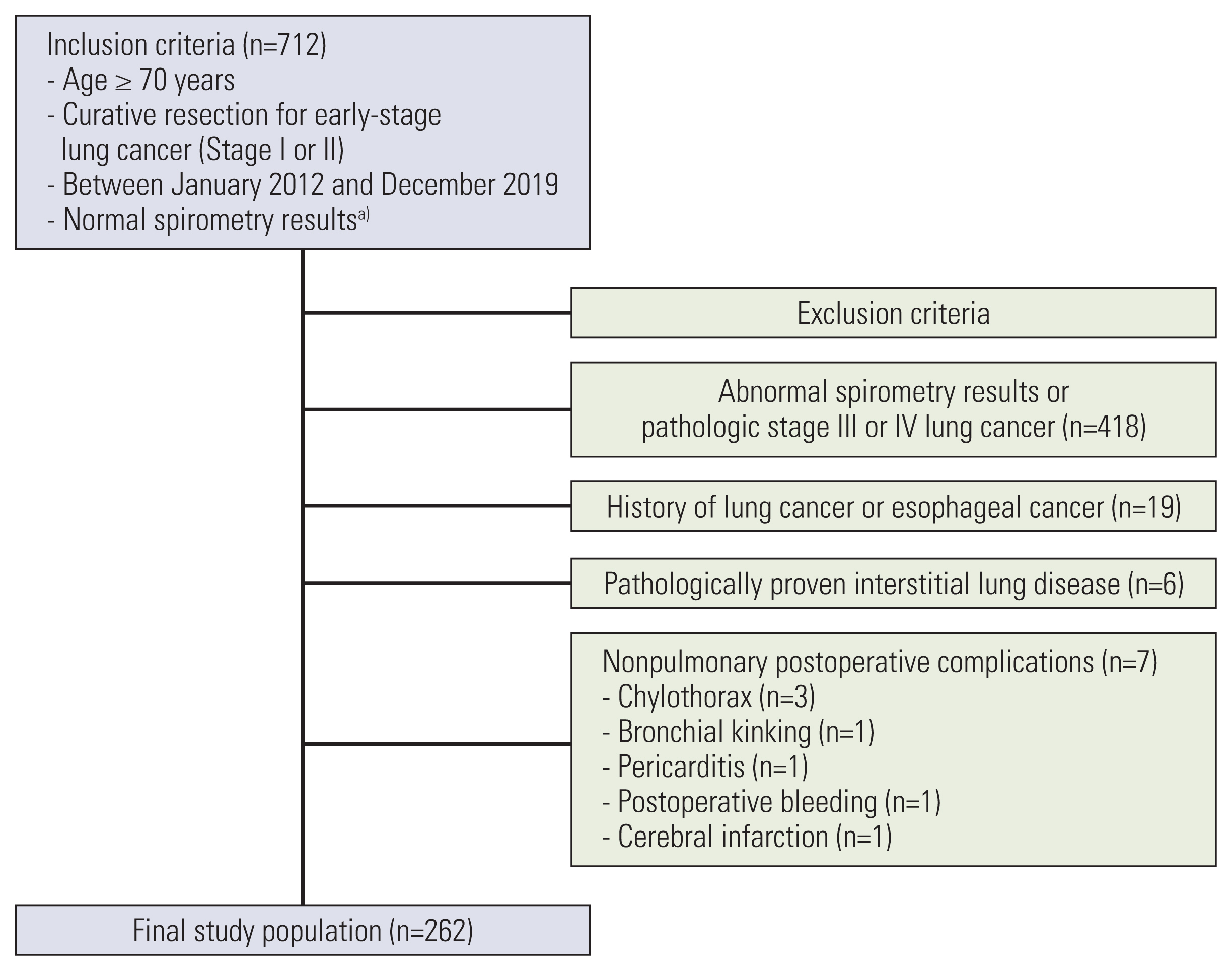
Flowchart of patient selection and study inclusion and exclusion criteria. a)A pre-bronchodilator forced expiratory volume in 1 second to forced vital capacity (FVC) ratio of > 0.70 and FVC ≥ 80% of the predicted value.
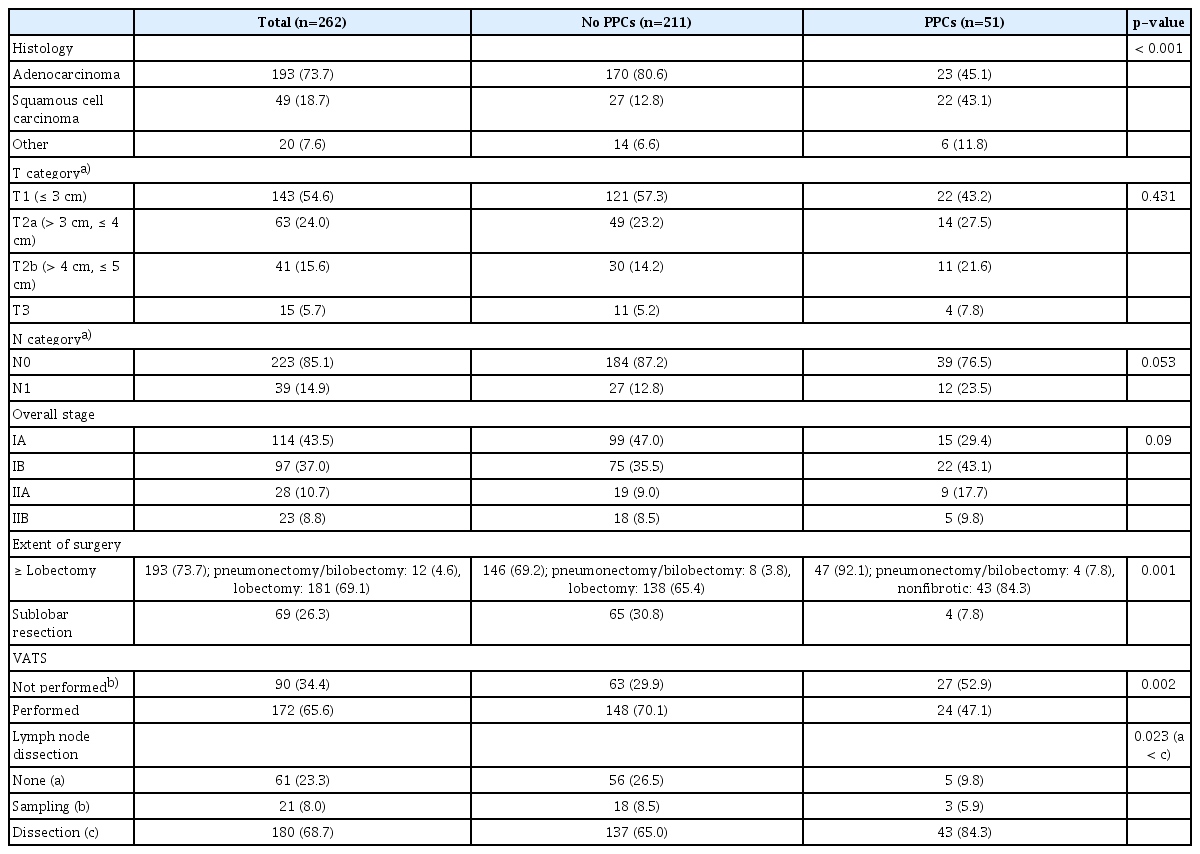
The median (interquartile range) age of the patients was 73 (71–76) years. Among the 262 patients, 132 were men (50.4%). The numbers of current and former smokers were 27 (10.3%) and 89 (34%), respectively. Fifty patients (19%) had ASA classification ≥ 3. Diabetes mellitus (31.3%) and hypertension (55.7%) were common comorbidities. The median (interquartile range) serum hemoglobin and serum albumin levels were 13 (12.1–14.1) g/dL and 4.4 (4.2–4.6) g/dL, respectively. The median (interquartile range) percentages of predicted pre-bronchodilator FEV1 and DLCO were 99% (91%–108%) and 102% (86%–117.1%), respectively. Bronchial wall thickening (32.1%), ILAs (29%), and emphysema (29.4%) were common preoperative CT findings. Among the ILA subtypes, subpleural nonfibrotic ILAs (16.4%) were the most common, followed by subpleural fibrotic (8.8%) and nonsubpleural (3.8%) ILAs. The two radiologists showed substantial interobserver agreement (κ=0.69; 95% CI, 0.61 to 0.78; p < 0.001) (Table 1). Adenocarcinoma and squamous cell carcinoma accounted for 73.7% and 18.7% of the cases, respectively. Most patients underwent lobectomy (69.1%) and systematic lymph node dissection (68.7%). Approximately two-thirds of all surgeries were video-assisted thoracic surgeries (65.6%) (Table 2).
A total of 51 patients (19.5%) showed PPCs. Air leak was the most common (29/51, 56.9%) among all PPCs, followed by pneumonia (18/51, 35.3%). Fig. 3 shows the PPCs in detail. Male sex (p < 0.001), current and former smoking (p < 0.001), ASA ≥ 3 (p=0.001), lower serum albumin level (p=0.019), and lower percentage predicted DLCO (p < 0.001) were significantly associated with PPCs in the univariable analysis. Among the preoperative CT findings, fibrotic ILAs (p < 0.001) and emphysema (p < 0.001) were significantly associated with PPCs. Patients with squamous cell carcinoma and other NSCLCs were more likely to have PPCs than those with adenocarcinoma (p < 0.001). The clinical T and N categories were not associated with PPCs. Patients with PPCs were more likely to have undergone lobectomy or more extensive resection (p=0.001), open thoracotomy (p=0.002), and systematic lymph node dissection (p < 0.001).

Histogram of postoperative pulmonary complications. Some patients had two or more complications. ARDS, acute respiratory distress syndrome; BPF, bronchopleural fistula; ILA, interstitial lung abnormality.
A multivariable logistic regression model revealed that, among several relevant risk factors, ASA ≥ 3 (adjusted odds ratio [OR], 2.53; 95% CI, 1.03 to 6.2; p=0.043), fibrotic ILAs (adjusted OR, 4.84; 95% CI, 1.35 to 17.38; p=0.016), and lobectomy or more extensive resection (adjusted OR, 10.39; 95% CI, 2.77 to 39.04; p=0.001) independently predicted PPCs. Moreover, male sex (adjusted OR, 15.49; 95% CI, 1.19 to 200.97; p=0.036), fibrotic ILAs (adjusted OR, 8.72; 95% CI, 1.71 to 44.38; p=0.009), and lobectomy or more extensive resection (adjusted OR, 9.66; 95% CI, 1.01 to 92.42; p=0.049) were significantly associated with major PPCs (Table 3).
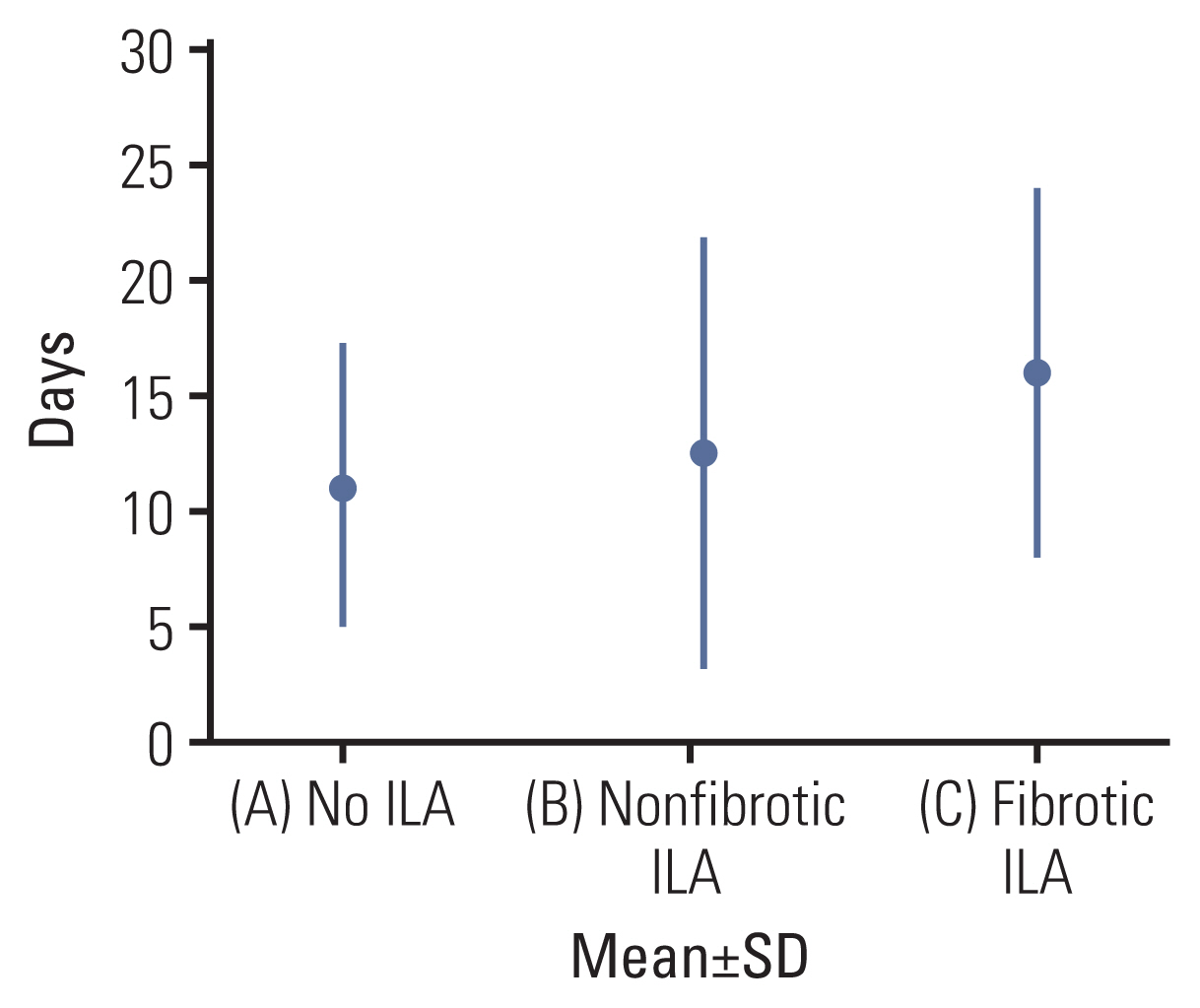
Patients with fibrotic ILAs showed significantly higher 30-day mortality than those without ILAs and higher 90-day mortality than those without ILAs or with nonfibrotic ILAs. The 180-day mortality did not show statistical significance. An ANOVA was performed for the length of hospital stay according to ILA subtypes (Table 4). Patients with fibrotic ILAs showed significantly longer length of hospital stay than those without ILAs (F=5.21, p=0.006) (Table 5, Fig. 4).
Discussion
ILAs are incidental radiologic findings in the spectrum of pulmonary fibrosis [13]. Older age, male sex, and smoking history are well-known risk factors [16]. Patients with ILAs may have mild respiratory symptoms and physiologic dysfunction, but not as much as patients with ILD [17]. The difference between ILA and ILD is the presence of clinically significant findings and physiologic dysfunction in ILD. Among the subclassifications of ILAs, fibrotic ILAs are particularly considered an important precursor of idiopathic pulmonary fibrosis and have several clinical implications [13]. Patients with fibrotic ILAs show high rates of progression and all-cause mortality [18,19]. The proportion of fibrotic ILAs in our study (23/76, 30.3%) was consistent with that in a previous study in which fibrotic ILAs accounted for 30% of all ILAs in lung cancer screening participants [18]. Fibrotic ILAs have prognostic relevance in patients undergoing lung cancer treatment. Iwasawa et al. [20] used a computer-aided detection system for detecting ILAs and assessing the percentage fibrosis extent. They concluded that the percentage fibrosis extent on preoperative CT was significantly correlated with not only the presence and severity of ILAs but also lower disease-free survival. Im et al. [21] reported that the ILA extent was significantly associated with PPCs. Our study showed that patients with fibrotic ILAs were more likely to have both overall PPCs and major PPCs than those without ILAs. ILD is generally known as a risk factor for PPCs in patients undergoing lung cancer resection or emergency surgery [22]. As fibrotic ILAs are believed to be a precursor of idiopathic pulmonary fibrosis, patients with fibrotic ILAs may show a higher incidence of PPCs. Additionally, it has been reported that ILAs are associated with fibrosis on pathologic correlation. Generally, ILAs are correlated with various interstitial pathologic conditions, including benign interstitial abnormalities, smoking-related interstitial fibrosis, and early-stage idiopathic pulmonary fibrosis [23]. Miller et al. [24] reported that subpleural ILAs especially show a significant correlation with subpleural fibrosis and other usual findings related to interstitial pneumonia on histopathology. This may explain the prognostic implication of fibrotic ILAs in our study. When evaluating patients who are planning to undergo lung cancer surgery, assessment and subclassification of ILAs detected on preoperative CT are essential because ILAs can lead to unfavorable clinical outcomes. Modification of risk factors, such as smoking cessation and active implementation of pulmonary rehabilitation including physiotherapy, can help reduce and manage PPCs in patients with ILAs.
The incidence of ILAs in our study was 29%, which is higher than that in previous reports [9,20]. The high incidence of ILAs in our study can be explained by several reasons. First, we did not include equivocal ILA as a category in this study. Focal or unilateral ground-glass or reticular abnormalities were considered equivocal ILAs in previous reports [18,20]. Although prone-position CT is known to be helpful in differentiating dependent lung opacity from an ILA, preoperative CT before lung cancer surgery is generally performed in the supine position; thus, dependent lung opacity may have been included as an ILA in this study. Despite these limitations, we attempted to identify ILAs based on the existence of extensive unilateral abnormalities and additional radiologic findings such as the presence of mild traction bronchiectasis within an ambiguous CT finding. The Fleischner Society guidelines also suggest that only mild unilateral abnormality is not considered an ILA. Second, our study population comprised elderly patients (age ≥ 70 years). As age is one of the major risk factors for ILA, this may have contributed to the higher incidence of ILAs in our study than in other studies.
Operative mortality, including 30- and 90-day mortality, reflects procedure-related death in patients undergoing major resections for thoracic malignancies [25]. Operative mortality is an important concept because it is an indicator of overall quality control. In general, patients undergoing surgery for lung cancer have a relatively low chance of overcoming adverse events after surgery because of old age and many comorbidities. ILD is a risk factor for operative mortality in patients undergoing NSCLC surgery. Patients with underlying ILD undergoing NSCLC surgery have a poor survival expectancy [26]. Fibrotic ILAs predicted significantly higher 30- and 90-day mortality than nonfibrotic ILAs and no ILAs. The 180-day mortality did not show statistical significance, which can be explained by the fewer numbers of death by 180 days after surgery (8/262).
The length of hospital stay is also a predictor of postoperative recovery. Various patient-related, surgery-related, and socioeconomic factors are associated with the hospital stay duration. PPCs are among the representative factors affecting hospital stay [27]. The length of hospital stay is associated with hospital costs and hospital-acquired complications [28]. Patients undergoing lung cancer surgery are at a risk of experiencing adverse events because lung cancer mainly occurs in older people who have many comorbidities. By reducing the length of hospital stay after lung cancer surgery, the accompanying complications can also be reduced. This study revealed that the hospital stay duration was longer in patients with fibrotic ILAs than in those without ILAs. Preoperative fibrotic ILAs on CT may serve as a prognostic indicator of the length of hospital stay. Providing an enhanced recovery pathway for patients planning to undergo elective lung cancer surgery could reduce the length of hospital stay by promoting early discharge [29].
This study had several limitations. First, this was a retrospective study based on medical records. Second, this was a single-center study, which limits the external validation of the results. Lung distortion as one of the major definitions of fibrotic ILAs in the Fleischner Society guidelines is ambiguous, which could lead to discordant subclassifications by different readers. We conducted this study simply dichotomously with respect to emphysema and ILAs. Nowadays, quantitative analysis with new techniques is helpful in disease diagnosis, prognosis prediction, and longitudinal management of diffuse lung disease [30]. Quantitative analysis of the extent of pulmonary involvement may yield more meaningful results, and more studies with quantification are needed. Moreover, ILAs have a variety of imaging features. Which specific imaging features influence the occurrence of PPCs need to be identified. In addition, the ILAs in this study were not pathologically proven. Despite these limitations, this study proved that fibrotic ILAs are independent predictors of overall PPCs and major PPCs.
In conclusion, fibrotic ILAs on preoperative CT are associated with PPCs, higher operative mortality, and longer length of hospital stay in elderly patients undergoing curative resection for early-stage lung cancer. Detecting fibrotic ILAs will allow clinicians to identify in advance patients with a high risk of developing PPCs, which will enable enhanced and efficient patient management through intensive postoperative care.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
Notes
Ethical Statement
This retrospective study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (approval no. CNUHH-2021-020). Informed consent was waived due to retrospective study design.
Author Contributions
Conceived and designed the analysis: Jeong WG, Kim YH, Lee JE.
Collected the data: Jeong WG, Lee JE.
Contributed data or analysis tools: Jeong WG, Lee JE, Oh IJ, Song SY, Chae KJ, Park HM.
Performed the analysis: Jeong WG.
Wrote the paper: Jeong WG.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.