Postmastectomy Radiation Therapy for Node-Negative Breast Cancer of 5 cm or Larger Tumors: A Multicenter Retrospective Analysis (KROG 20-03)
Article information
Abstract
Purpose
This study aimed to evaluate the role of postmastectomy radiation therapy (PMRT) in patients with node-negative breast cancer of 5cm or larger tumors undergoing mastectomy.
Materials and Methods
Medical records of 274 patients from 18 institutions treated with mastectomy between January 2000 and December 2016 were retrospectively reviewed. Among these, 202 patients underwent PMRT, while 72 did not. Two hundred and forty-one patients (88.0%) received systemic chemotherapy, and 172 (62.8%) received hormonal therapy. Patients receiving PMRT were younger, more likely to have progesterone receptor-positive tumors, and received adjuvant chemotherapy more frequently compared with those without PMRT (p < 0.001, p=0.018, and p < 0.001, respectively). Other characteristics were not significantly different between the two groups.
Results
With a median follow-up of 95 months (range, 1 to 249 months), there were nine locoregional recurrences, and 20 distant metastases. The 8-year locoregional recurrence-free survival rates were 98.0% with PMRT and 91.3% without PMRT (p=0.133), and the 8-year disease-free survival (DFS) rates were 91.8% with PMRT and 73.9% without PMRT (p=0.008). On multivariate analysis incorporating age, histologic grade, lymphovascular invasion, hormonal therapy, chemotherapy, and PMRT, the absence of lymphovascular invasion and the receipt of PMRT were associated with improved DFS (p=0.025 and p=0.009, respectively).
Conclusion
Locoregional recurrence rate was very low in node-negative breast cancer of 5 cm or larger tumors treated with mastectomy regardless of the receipt of PMRT. However, PMRT was significantly associated with improved DFS. Further investigation is needed to confirm these findings.
Introduction
Postmastectomy radiation therapy (PMRT) reduces loco-regional recurrences (LRR) and increases survival in pT3–4 and/or node-positive breast cancer [1,2]. However, in the intermediate-risk group including pT3N0 disease, the role of PMRT has been controversial. According to the National Comprehensive Cancer Network (NCCN) guidelines, the panels suggest ‘consider RT’ in pT3N0 breast cancer as category 2A [3]. In contrast, Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis consistently showed that there was no survival benefit of PMRT in node-negative disease [4,5].
Due to the low incidence of pT3N0 disease, there are no high level evidence to support the routine use of PMRT. There are a few studies of population- or hospital-based database suggesting survival benefit of PMRT [6–8], while contradictory data also exist [9,10]. In the meanwhile, American Society of Clinical Oncology (ASCO), American Society for Radiation Oncology, and Society of Surgical Oncology published the focused guideline update on PMRT, but issues regarding pT3N0 were not included [11].
In this study, we evaluated the role of PMRT in patients with node-negative breast cancer of 5 cm or larger tumors undergoing mastectomy via a multicenter retrospective study.
Materials and Methods
1. Study population
The medical records of patients with node-negative breast cancers of 5cm or larger tumor treated with mastectomy between January 2000 and December 2016 reviewed and retrospectively analyzed. Patients receiving neoadjuvant chemotherapy or those without detailed information on radiotherapy (RT) were excluded.
Pathologic information such as histologic subtype, histologic grade, lymphovascular invasion (LVI), resection margin status, and immunohistochemical staining of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) were retrieved from the reports of each institution.
2. Statistical analysis
Local recurrences were defined as tumor recurrences in the ipsilateral chest wall, and regional recurrences as those in the ipsilateral axillary, supraclavicular, and/or internal mammary nodes. Distant metastases were defined as disease recurrences other than local and/or regional recurrences. The time interval between surgery and LRR was measured as LRR-free survival (LRRFS). And, the time interval between surgery and recurrence, death, or last follow-up was measured as disease-free survival (DFS). Categorical variables were compared using chi-square test or Fisher’s exact test, and continuous variables using t test or Mann-Whitney U test. The actuarial survival rates were calculated using the Kaplan-Meier method. Log-rank test was used for univariate analysis, and Cox proportional-hazard model was used for multivariate analysis incorporating factors with a p-value < 0.1 on univariate analysis. Variables with a statistically different distribution between treatment groups were also included in the multivariate analysis. All statistical analyses were performed using PAWS Statistics for Windows ver. 18.0 (SPSS Inc., Chicago, IL).
Results
1. Characteristics
A total of 274 patients from 18 institutions were accrued. The median age was 49 years (range, 28 to 88 years). Tumor size ranged from 5 to 17 cm (median, 6 cm). Histologic subtype was invasive ductal carcinoma in 181 patients (66.1%), invasive lobular carcinoma in 42 (15.3%), and others in 51. Histologic grade 3 was observed in 113 patients (41.2%), and LVI in 69 (25.2%). Resection margin was involved in seven patients (2.6%), and all of these patients received PMRT. ER was positive in 165 patients (60.2%), and HER2 status was positive in 55 patients (20.1%).
Patient and tumor characteristics according to the receipt of PMRT were summarized in Table 1. The median age was younger and PR-positive tumors were more common in the PMRT group (p < 0.001 and p=0.018, respectively). Regarding systemic therapy, adjuvant chemotherapy was more frequently given to those patients of the PMRT group (p < 0.001). Other variables were not significantly different between the two groups.
2. Treatment
Total mastectomy was performed in most patients (n=237), while skin sparing mastectomy or nipple sparing mastectomy were also included. Breast reconstruction was done in 63 patients. As for axillary surgery, axillary lymph node dissection (n=156) or sentinel lymph node biopsy (n=118) was performed.
Adjuvant chemotherapy was administered in 241 patients (88.0%): adriamycin-based regimen in 177, taxane-based regimen in 41, and others in 23. Hormonal therapy was given to 172 patients (62.8%). Anti-HER2 therapy was given to 21 of 55 patients with HER2-positive tumors. Twenty-eight of 34 patients not receiving anti-HER2 therapy were treated during the period before the reimbursement of anti-HER2 therapy in Korea.
Among 202 patients receiving PMRT, chest wall RT alone was given in 76 patients, chest wall and supraclavicular node RT in 82, and chest wall, supraclavicular, and internal mammary node RT in 44. All patients except nine received PMRT using a conventional fractionation. The total dose ranged from 40.05 to 64.4 Gy (median, 50.4 Gy).
3. Outcomes and prognostic factors
With a median duration of follow-up of 95 months, there were 9 LRR’s, 20 distant metastases, and 26 deaths. According to the receipt of PMRT, the number of LRR, distant metastasis, and death was five (2.5%), 11 (5.4%), and 16 (7.9%) of 202 patients receiving PMRT, while four (5.6%), nine (12.5%), and 10 (13.9%) of 72 patients not receiving PMRT. Regarding LRR, three patients had simultaneous regional recurrences and distant metastases, and another three patients with local or regional recurrences had subsequent distant metastases. Detailed information of LRR’s were summarized in Table 2.
PR-positive tumors (p=0.053) and the receipt of hormonal therapy (p=0.051) were associated with a higher LRRFS, although the statistical significance was not reached. According to the receipt of PMRT, the 8-year LRRFS rates were not different between the two groups: 98.0% with PMRT and 91.3% without PMRT (p=0.133) (Fig. 1). Univariate analysis for LRRFS was presented in Table 3, but multivariate analysis could not be performed due to the small number of events.
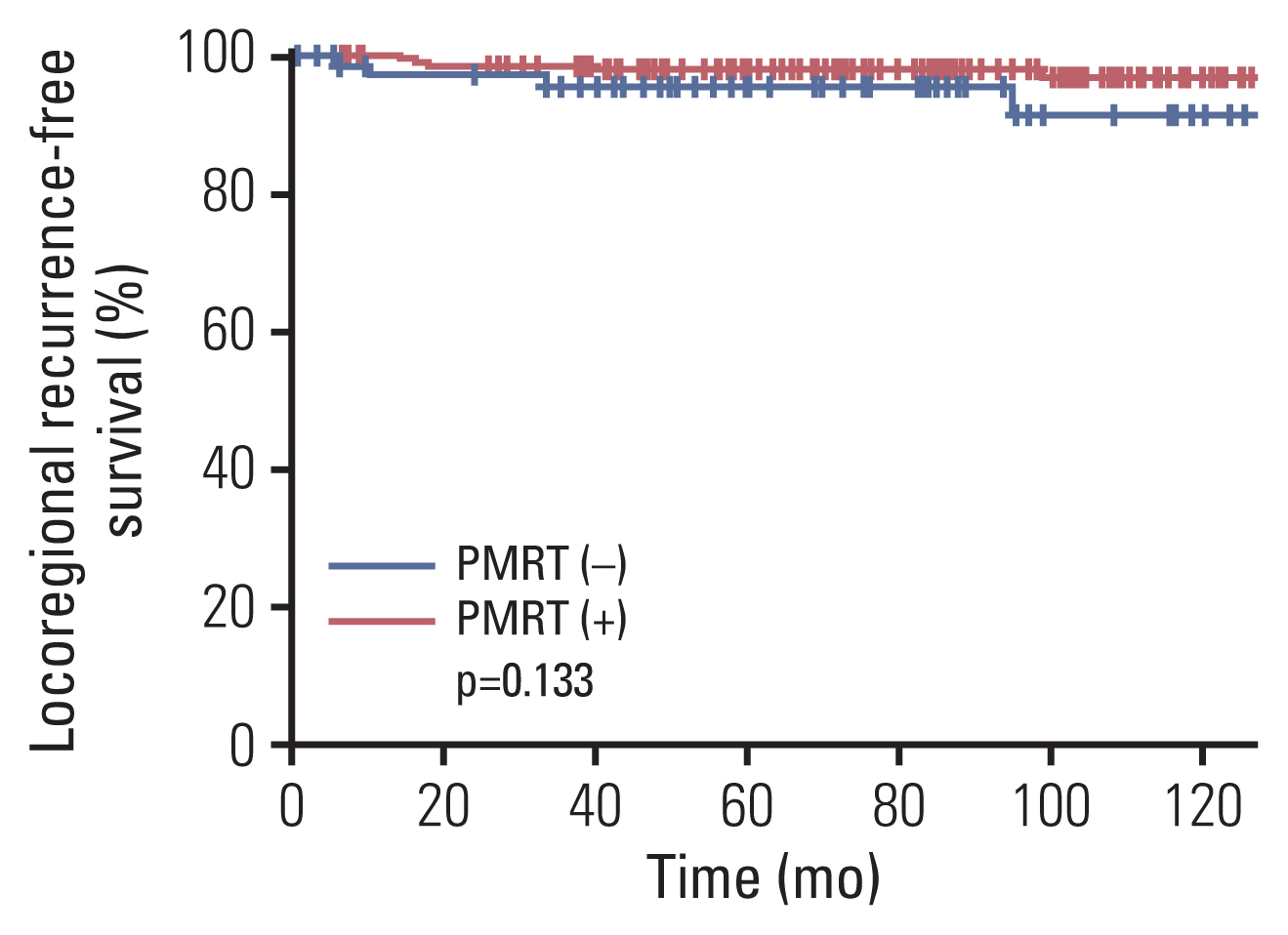
Locoregional recurrence-free survival curves according to the receipt of postmastectomy radiation therapy (PMRT).
As for DFS, ER status, histologic grade, hormonal therapy, and PMRT were correlated with DFS (p=0.027, p=0.025, p=0.032, and p=0.008, respectively), while the statistical significance for LVI was marginal (p=0.066). When age, histologic grade, LVI, chemotherapy, hormonal therapy, and PMRT were incorporated into multivariate analysis, the absence of LVI and the receipt of PMRT were associated with improved DFS (p=0.025 and p=0.009, respectively) (Table 4). Patients receiving PMRT had a higher 8-year DFS rate of 91.8% compared to 73.9% of those without PMRT (p=0.008) (Fig. 2). Although patients in the PMRT group received adjuvant chemotherapy more frequently (p < 0.001), chemotherapy was not associated with DFS on both univariate and multivariate analyses (p=0.800 and p=0.657, respectively).
Discussion
The role of PMRT in intermediate-risk breast cancer has been controversial. Recently, two landmark randomized trials proved the benefit of regional nodal RT in early stage node-positive breast cancers [12,13]. Because the indication of regional nodal RT is in line with that of PMRT, pT1-2N1 breast cancer has been increasingly considered as the candidate for PMRT. However, regarding pT3N0 cases, there are a paucity of studies investigating the role of PMRT except for a series of studies using population- or hospital-based database from the United States (Table 5).

Summary of recent studies on PMRT in patients with node-negative breast cancer of 5 cm or larger tumors
Johnson et al. [6] analyzed 2,525 patients with pT3N0 breast cancer undergoing mastectomy from Surveillance, Epidemiology, and End Results (SEER) database, and showed that 42% of these patients received PMRT and that PMRT improved cancer-specific and overall survival. Using National Cancer Database (NCDB), Francis et al. [7] also demonstrated that 47% of patients received PMRT, and overall survival was significantly increased by the addition of PMRT even after adjusting chemotherapy and hormonal therapy. Recently, however, it was noted that such a benefit was limited to those patients not receiving systemic chemotherapy [8]. In the current study, 88.0% of patients received systemic chemotherapy, and PMRT was associated with improved DFS. Among studies other than database-based ones, Goulart et al. [14] reported British Columbia experience showing the non-significant improvement of DFS in the PMRT group.
However, there are several studies reporting the absolute LRR rate is very low, which questioned the necessity of PMRT. Taghian et al. [9] already noted that the 10-year isolated LRR rate was as low as 7.1% in node-negative patients with 5 cm or larger tumors undergoing mastectomy without PMRT. And, when systemic chemotherapy and/or hormonal therapy was given, the incidences decreased to around 5%. Floyd et al. [10] also reported a similar finding among patients receiving systemic therapy, in which the 5-year LRR rate was 7.6%. Similarly, in our study, the LRR rate without PMRT was 8.7% at 8 years, and DFS benefit of PMRT seemed to be originated from the reduction of distant meta-stasis and death rather than LRR. However, LRR might be underestimated in the presence of distant metastasis. It was also reported that the landmark randomized trials demonstrated regional nodal irradiation significantly reduced the risk of distant metastasis [12,13], which suggested that LRR rate should not be used as the sole indication for PMRT. Although no risk factors predicting LRR were identified in the study of Taghian et al. [9], LVI was correlated with LRRFS and DFS in the study of Floyd et al. [10]. In the present study, LVI was also associated with DFS. Regarding this issue, German guidelines recommend PMRT in pT3N0 disease with additional risk factors such as LVI, histologic grade 3, close resection margin, premenopausal women, and age < 50 years [15]. In contrast, a French group tested an index to identify the optimal candidate for PMRT in pN0-1mi patients [16]. This index considered six risk factors such as tumor size, histologic grade, LVI, age, ER, and HER2 status, but a high-risk group with three or more risk factors did not benefit from PMRT in their multicenter retrospective cohort. Given these observation, they concluded that decision making for PMRT should not be based on the number of such risk factors.
As for the patterns of failure, aforementioned two studies commonly indicated that the most frequent site of LRR was chest wall [9,10]. In contrast, seven of nine LRR occurred at regional nodes and no chest wall recurrence was observed in the PMRT group of our study. Therefore, optimal target volume of PMRT is another important issue for maximizing its therapeutic efficacy, if any. Cassidy et al. [17] analyzed 3,437 patients with pT3N0 breast cancer undergoing mastectomy from NCDB. Chest wall RT with or without regional nodal RT was associated with improved overall survival when compared with no RT, but additional regional nodal RT was not when compared with chest wall RT alone. In the current study, the greatest benefit in DFS was observed in patients receiving chest wall+supraclavicular+internal mammary node RT (data not shown) when PMRT group was separated into three subgroups according to the target volume. However, the number of patients per subgroup became much smaller, therefore definite conclusion could not be reached. Currently, one randomized controlled trial, SUPREMO, is underway [18]. The study population is the intermediate-risk breast cancer patients including pT3N0 as well as pT1-2N1 and pT2N0 tumors with grade 3 histology and/or LVI. However, the role of regional nodal RT cannot be evaluated because this study compares chest wall RT alone versus no RT.
There are a number of limitations in our study. First, this is a retrospective study, which is prone to selection biases. Patients of the PMRT group were younger and more likely to receive chemotherapy than those of no PMRT group. However, when age and chemotherapy was incorporated into the multivariate analysis, these factors were not correlated with DFS. Nevertheless, performance status which was unavailable in our study might have an influence on the treatment outcomes. Second, the number of patients and observed events was small, and this could contribute the non-significant correlation between PMRT and LRRFS despite these patients were accrued from 18 institutions over 16 years. Given the widespread adoption of neoadjuvant chemotherapy in operable breast cancer, patients within this category would be further decreased in the future. Lastly, the heterogeneous treatments including chemotherapeutic regimens and RT target volumes might also affect the treatment outcomes.
In conclusion, absolute LRR rate was very low in node-negative breast cancer of 5cm or larger tumors undergoing mastectomy with or without PMRT. However, PMRT was significantly associated with improved DFS. Further studies are warranted to confirm these findings, and optimal target volume issue also needs to be addressed.
Notes
Ethical Statement
The institutional review board of each institution approved this study (approval number: H-2005-179-1126 at Seoul National University Hospital), and waived the requirement for obtaining informed consent.
Author Contributions
Conceived and designed the analysis: Kim K, Shin KH.
Collected the data: Kim K, Jung J, Kim H, Jung W, Shin KH, Chang JH, Kim SS, Park W, Chang JS, Kim YB, Ahn SJ, Lee IK, Lee JH, Park HJ, Cha J, Kim J, Choi JH, Koo T, Kwon J, Kim JH, Kim MY, Park SH, Kim YJ.
Contributed data or analysis tools: Shin KH.
Performed the analysis: Kim K.
Wrote the paper: Kim K, Jung J, Kim H, Jung W, Shin KH, Chang JH, Kim SS, Park W, Chang JS, Kim YB, Ahn SJ, Lee IK, Lee JH, Park HJ, Cha J, Kim J, Choi JH, Koo T, Kwon J, Kim JH, Kim MY, Park SH, Kim YJ.
Conflicts of Interest
Conflict of interest relevant to this article was not reported.





