Clinicopathologic Features and Long-Term Outcomes of Elderly Breast Cancer Patients: Experiences at a Single Institution in Korea
Article information
Abstract
Purpose
The purpose of this study was to assess the tumor characteristics and long-term clinical outcomes of adjuvant treatments after surgery with a curative aim for patients with breast cancer who are 65 years and older.
Materials and Methods
Patients with breast cancer who underwent curative surgery from 2000 to 2009 were analyzed (n=4,388). Tumor characteristics and survival outcome were compared by dividing the patients into two age groups (< 65 and ≥ 65 years old). The Kaplan-Meier method was used for comparison of survival rates by log-rank test, and a Cox regression model was used to examine the effect of variables.
Results
Among 4,388 patients with invasive breast cancer, 317 patients (7.2%) were 65 years or older and the median age of all patients was 47 years (range, 18 to 91 years). Tumor characteristics were similar between the two age groups, but the older patients were treated less often with adjuvant treatments. During a median follow-up period of 122 months, recurrence-free survival (RFS) was equivalent for patients 65 years and older compared to younger patients, but significantly worse in overall survival (OS) and breast cancer–specific survival (BCSS) (5-year OS, 94.3% vs. 90.5%; p < 0.001 and 5-year BCSS, 94.7% vs. 91.8%; p=0.031). In the multivariate model, age ≥ 65 years old was identified as an independent risk factor for OS and RFS.
Conclusion
Elderly breast cancer appeared to have worse outcomes with very low prevalence in Korea, despite similar tumor characteristics. More active adjuvant therapies would have a role for aggressive subtypes for fit, elderly patients.
Introduction
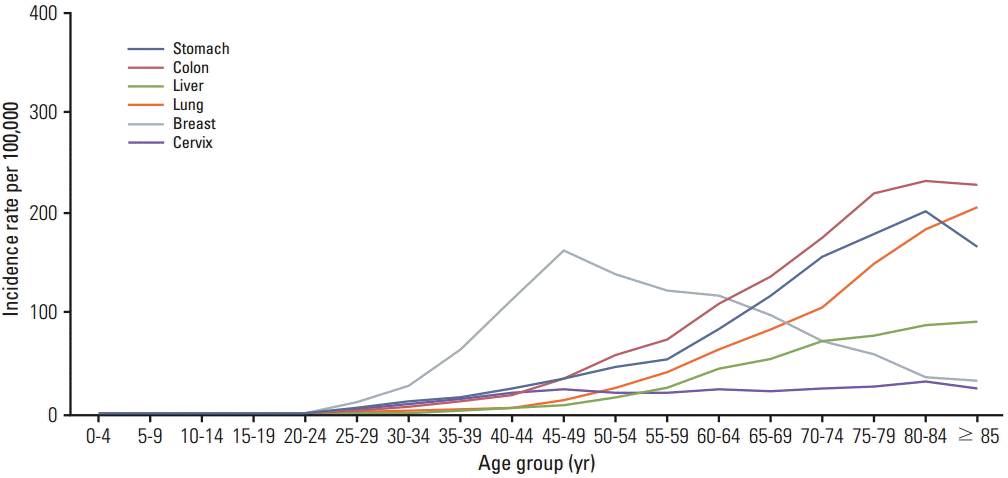
The elderly population is increasing worldwide. The population of 65 years or older among all cancer patients is anticipated to be 20.1% (70.2 million) of the total by 2030 in the United States [1], and 24.3% (12.6 million) in Korea [2]. Breast cancer is the most common cancer among women [3], and the number of older women with breast cancer is increasing. However, demographic features of patients with breast cancer differ between Asian and Western countries. Asian women, including Korean women, have a lower incidence rate and younger peak age of breast cancer compared with the Caucasian population [4,5]. The incidence of breast cancer in women peaks in their forties in Asia, but the peak age in the United States is in their sixties [6]. Fig. 1 shows that the incidence rates increase with age for most cancers, but that the peak incidence age of breast cancer was between 45 and 59 years in the Korean population [7].
As a consequence of the high incidence of young age breast cancer in Asia, a number of studies investigated young breast cancer including early stage breast cancer. However, older patients with breast cancer presented less with active treatments such as clinical trials [8]. Although most elderly patients with breast cancer present at an early stage [9], few studies on the clinical outcome and tumor biology of elderly patients with early stage breast cancer have been reported.
Comorbidity and functional status of elderly patients could result in poorer survival, but there are considerable factors that render positive outcomes in older patients. First, breast cancer in postmenopausal women shows a slow-growing and indolent nature [10]. Recently, the improvement of general health has increased the life expectancy to around 80 years in many countries, so that survival outcome could be comparable to that of younger patients in early stage breast cancer. However, the significance of early breast cancer on the survival of elderly patients is still unknown. Elderly breast cancer patients are defined as breast cancer patients aged 65 and over. We have chosen 65 years or older, however there is no consensus to define elderly breast cancer, because of the extremely low incidence for women 70 years or older in our patients’ cohort (3.6%, 159/4,388) and some geriatric recommendations [11,12].
Optimal treatment for older breast cancer patients is also not well established [13]. The treatment approach for elderly patients requires many considerations, including not only chronological age but also comorbidities, social and economic circumstances, and life expectancy. However, the majority of recommendations are based on retrospective analyses and the extrapolation of study results from younger patients. This may result in under treatment and poor survival outcomes in the older populations [14].
In this study, we have explored the biologic tumor characteristics and clinical outcomes of elderly women (65 years and older than 65 years of age) with early stage breast cancer. In addition, we compared the results with those of patients younger than 65 years old, with analysis of long-term outcome and survival rate. Then, our results were compared with those of Western countries.
Materials and Methods
1. Patients
In this retrospective cohort study, we collected data from the electronic medical records of patients diagnosed with invasive breast cancer who underwent curative surgery at Samsung Medical Center between 2000 and 2009. Data including TNM staging, the results of estrogen/progesterone/HER2 expression, nuclear grade, histologic grade, Ki-67 expression, lymphatic and vascular invasion, date of surgery, and all adjuvant therapies (including systemic chemotherapy, endocrine therapy, and radiotherapy) were collected for this study. The patients were divided into two age groups, < 65 years old and ≥ 65 years old. Intrinsic subtypes were classified according to four groups based on immunohistochemistry as follows: hormone receptor (HR)+/HER2–, HR+/HER2+, HR–/HER2+, and triple negative breast cancer. For detection of local or distant recurrence, clinical follow-up was performed every 3-6 months for the first 5 years after primary therapy and annually thereafter. Clinical follow-up included history-taking; physical examinations; laboratory tests, including carcinoembryonic antigen, cancer antigen 15-3, complete blood counts, and liver function tests; chest radiography; mammography; breast and abdominopelvic ultrasonography; and bone scans. In addition, a computed tomography (CT) scan, magnetic resonance imaging, or a fluorine-18 fluorodeoxyglucose positron emission tomography/CT scan was performed if necessary. This study was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Korea.
2. Statistical analysis
Differences in characteristics including distribution of intrinsic subtypes between two age groups (< 65 years vs. ≥ 65 years) were examined using Fisher exact test. Overall survival (OS) was measured from the date of curative surgery to the date of death or date the patient was last seen. Recurrence-free survival (RFS) was measured from the date of curative surgery to the date of breast cancer recurrence, regardless of whether recurrence was locoregional, contralateral breast and/or distant metastasis. Distant RFS (DRFS) was defined as the time from the date of curative surgery to the date of documented distant metastasis. Breast cancer-specific survival (BCSS) was determined from only breast cancer-specific death. The Kaplan-Meier method was used for estimation of OS, RFS, DRFS, and BCSS. Differences in survival were analyzed using the log-rank test and a p-value less than 0.05 was considered significant. A multivariable Cox proportional hazard regression model was used to assess the impact of the prognostic variable on OS and RFS. Data were analyzed using the IBM SPSS ver. 22.0 software (IBM Co., Armonk, NY).
Results
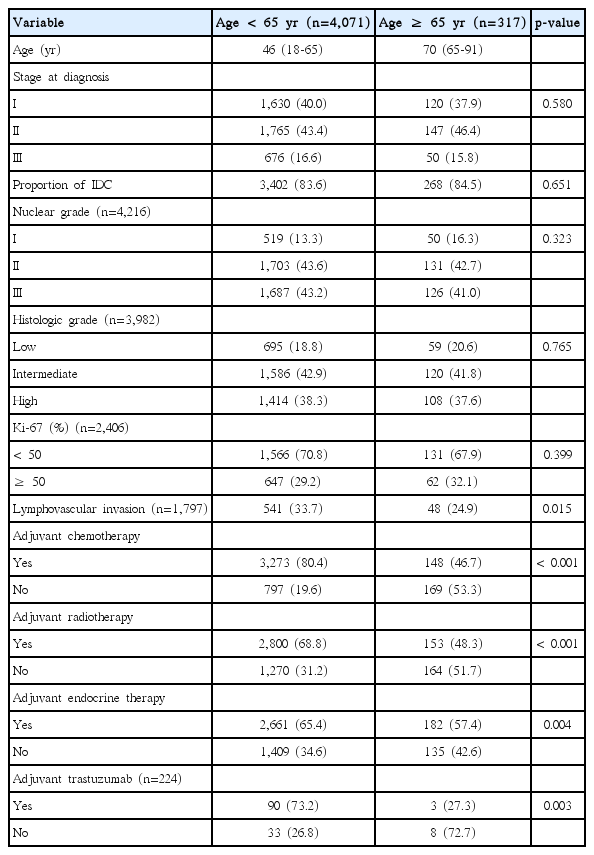
A total of 4,409 female patients who underwent curative surgery were diagnosed with invasive breast cancer between 2000 and 2009. Among them, 21 patients with metastatic breast cancer (stage IV) were identified and excluded from analysis. Only 317 patients (7.2%) were 65 years or older, while the rest of the patients were younger than 65 years old. The median ages of the younger and older groups were 46 years (range, 18 to 65 years) and 70 years (range, 65 to 91 years), respectively. There was no significant difference between the two groups according to TNM staging, nuclear grade, histologic grade, or Ki-67 expression. Lymphovascular invasion was more frequent in the younger age group (33.7% vs 24.9%, p=0.015). Older patients received adjuvant treatments, including local and systemic therapies for breast cancer, less often (Table 1). The majority of the younger age group were treated with adjuvant chemotherapy and radiotherapy (80.4% and 68.8%, respectively), whereas older patients were treated less often (46.7% and 48.3%, respectively). In the case of the adjuvant endocrine therapy, more than half of the older patients (51.7%) were prescribed adjuvant endocrine therapy. Patients with HER2+ had received adjuvant trastuzumab since 2008, because insurance did not reimburse the cost until that time. The majority of younger patients with HER2+ (73.2%) were treated with adjuvant trastuzumab, while only 27.3% of older patients received adjuvant trastuzumab treatment.
The 5-year RFS rate and OS rate in all patients (n=4,388) were 87.4% and 94.1%, respectively, with a median follow-up period of 122 months. Fig. 2 shows the Kaplan-Meier curves by RFS and DRFS, OS, and BCSS, according to age group. There were no significant differences in RFS and DRFS according to age group; however, the OS and BCSS differed between age groups. The results of univariate analysis for RFS and OS with a 5-year survival rate according to each variable are shown in Supplementary Tables 1 and 2.

Recurrence-free survival (RFS), distant recurrence-free survival (DRFS), overall survival (OS), and breast cancer-specific survival (BCSS) according to age groups.
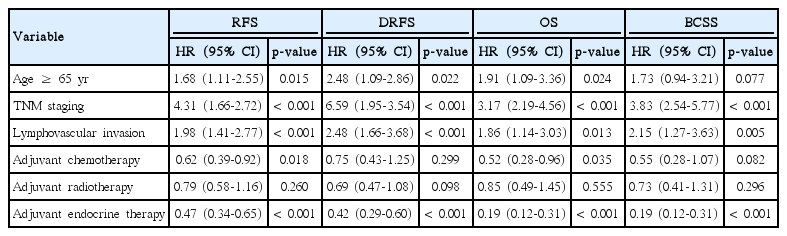
In multivariate Cox-regression analysis, old age (≥ 65 years), TNM staging, and lymphovascular invasion were identified as independent risk factors for RFS, DRFS, and OS. However, in terms of BCSS, old age was not an independent prognostic factor (hazard ratio, 0.079; 95% confidence interval, 0.94 to 3.21; p=0.079). Among the adjuvant therapies, adjuvant endocrine therapy was a positive prognostic factor in all survival analyses (Table 2).
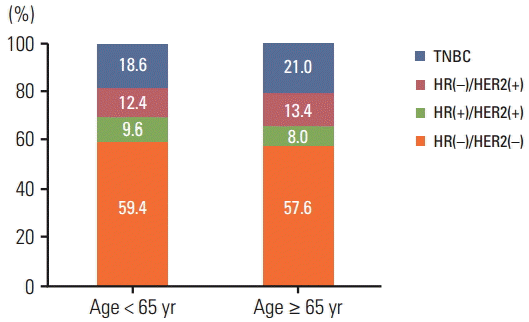
The distribution of intrinsic subtypes was almost identical between the two age groups (p=0.541), and the most common subtype was HR(+)/HER2(–), 59.4% and 57.6%, respectively (Fig. 3). In univariate analysis, intrinsic subtypes among younger patients (age < 65 years) were identified as risk factors in terms of RFS and OS (Supplementary Tables 1 and 2). Intrinsic subtypes were excluded as a variable in the multivariate Cox-regression analysis because it could be confounded with affected adjuvant endocrine therapy.
Discussion
Almost 50% of female patients with breast cancer are age 65 years or older in the United States with a median age of diagnosis at 61 years [15,16]. In the current study, this was in stark contrast with western countries where only 7.2% of patients in our cohort were 65 years or over, with the median age of all patients of 47 years (range, 18 to 91 years). Some differences between Asian and Western women, including breast density and HR, were identified [17], and further investigation of the clinical data regarding these differences is required.
Even though breast cancer in older women was known for its indolent nature [10,16], most tumor characteristics were similar between women in the two age groups in our study, except for lymphovascular invasion. The incidence of lymphovascular invasion was lower in older patients (24.9% vs. 33.7%, p=0.015); however, the evidence was too weak to support that breast cancer in older patients is less aggressive than in younger patients; this is because the other tumor characteristics, including nuclear grade, histologic grade, and expression of Ki-67, were not significantly different between the two age groups in this study.
This study showed that older patients were less likely to receive adjuvant treatments after curative surgery. The discrepancy between the two age groups was largest for adjuvant chemotherapy (48.3% vs. 80.4%, p < 0.001), because the comorbidity or disability in elderly patients with breast cancer poses a challenge for aggressive treatment. This finding is similar to those of studies in western countries [9,14,18]. However, endocrine therapy tended to be administered more often to older patients than other adjuvant therapies (57.4% vs. 65.4%, p=0.004), and adjuvant endocrine therapy was an independent, favorable prognostic factor in all survival analyses. Because the percentages of HRs were not significantly different between the two age groups, our findings suggest that all patients with breast cancer who have undergone curative surgery benefit from adjuvant endocrine therapy, regardless of age.
Several randomized trials confirmed the effectiveness of adjuvant chemotherapy and radiotherapy in improving survival for older women with early-stage breast cancer [19-21]. Under treatment of elderly breast cancer was demonstrated in several studies and typically resulted in worse clinical outcome [14,18]. Older patients in our cohort had less adjuvant therapies, and breast cancer–specific survival was lower than for younger patients (94.7% vs. 91.8% BCSS at 5 years, 89.5% vs. 85.7% BCSS at 10 years); this implies that age-adjusted BCSS was worse for older patients. In addition, age 65 years or older was an independent risk factor for OS, but not for BCSS in multivariate analysis. These findings support that older patients should receive the standard treatment as is typically given to younger patients when possible. However, the lack of clinical information on comorbidity and performance status for comprehensive geriatric assessment has weakened our results.
In the current study, it would appear that worse long-term OS in patients aged 65 years or older is related to increasing comorbidity and disability according to age. Differences between OS and BCSS in older patients also suggest that patients aged 65 or older die of causes other than their breast cancer. Functional status is a crucial factor for adjuvant treatments in the elderly and several scales of geriatric assessment have been developed [12,22,23]. The clinician should use these tools in identification of vulnerable patients from healthy patients and to avoid suboptimal treatments for fit, elderly patients. According to increased longevity, a large number of older women are in good health, thus they may benefit just as much from aggressive treatment as younger patients. In addition, this study confirms that the median age is still younger than those of Western countries, even in the older group in our patients’ cohort.
Conclusion
Only 7.5% of patients in our patients’ cohort were elderly breast cancer patients aged 65 years or older, and the tumor characteristics were similar between the two age groups (< 65 and ≥ 65 years old). However, most of the older patients were treated less often, even though they were in early stage of breast cancer. Further studies are needed to establish optimal treatment for elderly patients with breast cancer and to establish geriatric assessment for identification of high risk patients of treatment-related side effects. In addition, exploratory analysis for differentiation of biologic features between the two groups is warranted.
Electronic Supplementary Material
Supplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
Notes
Conflict of interest relevant to this article was not reported.