A Case of Gemcitabine and Cisplatin Associated Posterior Reversible Encephalopathy Syndrome
Article information
Abstract
A 58-year-old female receiving gemcitabine and cisplatin chemotherapy for stage IV gallbladder cancer developed the clinicoradiologic syndrome, posterior reversible encephalopathy syndrome (PRES). Just before the 4th gemcitabine chemotherapy cycle, she was admitted to the hospital with complaints of headache, dizziness, and generalized tonic-clonic seizures. A MRI was performed on the day when the seizure developed, and the findings showed patchy cortical and subcortical T2 hyperintensity without enhancement that involved both occipital and parietal lobes. Phenytoin loading and maintenance was started for prevention of recurrent seizures, which was successful. The follow-up brain MRI obtained 10 days after the seizure attack showed completely resolved radiologic findings. After the MRI findings revealed complete resolution, phenytoin maintenance was stopped. Even with discontinuation of phenytoin, she had no seizures or other clinical manifestations.
Introduction
Posterior reversible encephalopathy syndrome (PRES) is a reversible clinicoradiologic syndrome which presents with headaches, seizures, loss of vision, and altered mental status with typical imaging revealing primary involvement of the bilateral parietal-occipital lobes (1). Although the pathogenesis remains poorly understood, hypertensive encephalopathy, pre-eclampsia, eclampsia, renal failure, general anesthesia, and several immunosuppressant agents are known etiologic causes (1-4). Recently, several chemotherapeutic agents, such as cisplatin (5), cytarabine (6), and gemcitabine (7,8) have also been shown to cause PRES, thus physicians who are involved with chemotherapeutic agents need to manage patients with neurotoxicity related to chemotherapeutic agents. A few cases have been reported for chemotherapeutic agent-related PRES in Korea, but PRES is still uncommon (9-11). Herein we describe a patient who experienced PRES while receiving gemcitabine and cisplatin combination chemotherapy for gallbladder cancer.
Case Report
A 58-year-old woman was diagnosed with gallbladder cancer had hepatic and regional lymph node metastases. Palliative capecitabine chemotherapy was begun; however, the disease progressed with aggravated lung metastases during the 2nd cycle. The chemotherapy regimen was changed to gemcitabine (1,200 mg/m2 on days 1 and 8 of each 21-day cycle) and cisplatin (60 mg/m2 on day 1 of each 21-day cycle). Her performance status remained excellent. Two weeks after the 3rd cycle of chemotherapy, she was admitted with the sudden onset of headaches, dizziness, and generalized tonic-clonic seizures. At that time, her blood pressure was 170/90 mmHg, while her blood pressure was 100~150/50~80 mmHg during previous hospitalizations. A head computed tomography was obtained initially, which showed focal low density in both occipital lobes without any evidence of brain metastasis.
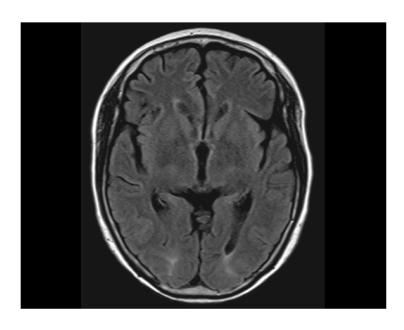
Brain magnetic resonance imaging (MRI; T2 and fluid-attenuated inversion recovery [FLAIR] image sequences) was obtained and revealed patchy cortical and subcortical T2 hyperintensity without enhancement, apparent on both occipital and parietal lobes (Fig. 1). The clinicoradiographic diagnosis was consistent with posterior reversible encephalopahty syndrome (PRES). With phenytoin loading and maintenance therapy, her mental status gradually normalized. A follow-up MRI obtained 10 days after the seizure attack showed completely resolved radiologic findings, which was consistent with the diagnosis of PRES (Fig. 2). While receiving supportive care during the hospital stay, she received no more chemotherapy. With improved cognition, she was discharged from the hospital, but approximately 1 month after discharge, she ultimately succumbed of cancer progression.
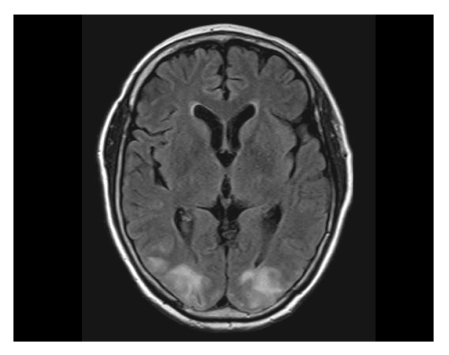
Patchy cortical and subcortical T2 hyperintensity without enhancement, bilateral occipital and parietal lobes (on the day of seizure attack [7/31/2008]).
Discussion
PRES is a clinicoradiologic entity characterized by headaches, altered mental status, seizures, and visual loss. PRES was first described by Hinchey et al. (1) in 1996. He offered the term reversible posterior leukoencephalopathy syndrome (RPLS) for a series of patients who presented with headaches, altered mental status, seizures, and loss of vision, and radiologic findings of reversible symmetric posterior cerebral white-matter abnormalities on CT and MRI. Although the pathogenesis remains unclear, several conditions, including hypertensive encephalopathy, pre-eclampsia/eclampsia, renal failure, general anesthesia, immunosuppressants, and cytotoxic agents (1-8), have been shown to cause PRES. The suggested pathophysiologic mechanisms are cerebral vasospasm with resulting ischemia within the involved territories and a breakdown in cerebrovascular autoregulation with ensuing interstitial extravasation of fluid (12). Radiographic and clinical findings are typically reversible within 2 weeks after correction of the precipitating event. Otherwise, persistent neurologic defects may occur with progression to infarction, hemorrhage, or death.
A recent study reviewed the 22 Korean cases who presented with PRES (13). Pre-eclampsia was the leading cause of PRES, followed by immunosuppressants, renal failure, and migraine. With respect to chemotherapeutic agent-associated PRES, a few cases of leukoencephalopathy associated with 5-FU, cisplatin, and cytarabine have been reported in Korea (9-11).
Gemcitabine-associated PRES was first identified in 2001 (7). After this, a few reports followed and the authors suggested neurotoxicity of gemcitabine by showing that PRES re-developed after re-administration of gemcitabine (8,14). Although the precise mechanism of PRES by chemotherapeutic agents, such as cisplatin, cytarabine, gemcitabine, interferon-a, rituximab, or sorafenib remains unclear, the brain-capillary leak syndrome related to hypertension or direct CNS microvascular injury has been suspected (4-8,15). With respect to the cisplatin-induced encephalopathy, hypomagnesemia has been suggested to be a contributing factor for PRES (5).
Gemcitabine and/or cisplatin are commonly used drugs in many solid cancers. We hereby present PRES from frequently used chemotherapeutic drugs. The time to onset of PRES by those agents can be within several hours to 3 months after repeated infusion. Because our patient received the combination chemotherapy with gemcitabine and cisplatin, no one can be certain about which agent was the main causative factor of PRES.
PRES is reversible with discontinuation of causative agents and supportive management; however, if undetected, PRES may lead to permanent neurologic damage or death. Thus, increasing awareness of PRES is needed in patients who undergo chemotherapy with those agents. Further investigations into the mechanisms of toxicity and risk factors of PRES caused by chemotherapy are required to prevent unexpected complications.