A New Isolated Mediastinal Lymph Node or Small Pulmonary Nodule Arising during Breast Cancer Surveillance Following Curative Surgery: Clinical Factors That Differentiate Malignant from Benign Lesions
Article information
Abstract
Purpose
A newly isolated mediastinal lymph node (LN) or a small pulmonary nodule, which appears during breast cancer surveillance, may pose a diagnostic dilemma with regard to malignancy. We conducted this study to determine which clinical factors were useful for the differentiation of malignant lesions from benign lesions under these circumstances.
Materials and Methods
We enrolled breast cancer patients who were presented with a new isolated mediastinal LN or small pulmonary nodule that arose during surveillance, and whose lesions were pathologically confirmed. Tissue diagnosis was made by mediastinoscopy, video-assisted thoracic surgery or thoracotomy.
Results
A total of 43 patients were enrolled (mediastinal LN, 13 patients; pulmonary nodule, 30 patients). Eighteen patients (41.9%) were pathologically confirmed to have a benign lesion (benign group), and 25 patients (58.1%) were confirmed to have malignant lesion (malignant group). Between the two groups, the initial tumor size (p=0.096) and N stage (p=0.749) were similar. Hormone receptor negativity was more prevalent in the malignant group (59.1% vs. 40.9%, p=0.048). The mean lesion size was larger in the malignant group than in the benign group (20.8 mm vs. 14.4 mm, p=0.024). Metastatic lesions had a significantly higher value of maximal standardized uptake (mSUV) than that of benign lesions (6.4 vs. 3.4, p=0.021).
Conclusion
Hormone receptor status, lesion size, and mSUV on positron emission tomography are helpful in the differentiation of malignant lesions from benign lesions in breast cancer patients who were presented with a new isolated mediastinal LN or small pulmonary nodule during surveillance.
Introduction
Breast cancer is the most common cancer in women worldwide, affecting approximately 240,000 cases in North America [1]. Lung, bone, and brain are common sites of metastasis in patients with breast cancer [2]. Mediastinal lymph node (LN) metastases, however, occur in only 2% of patients with breast cancer during surveillance [3]. A mediastinal LN enlargement or small pulmonary nodule is frequently imaged via computed tomography (CT) scan during surveillance. If a mediastinal LN enlargement or small pulmonary nodule is suspicious for malignancy, it is essential to obtain adequate tissue from the lesion to perform a pathological diagnosis since further treatment plans are dependent on these results. If the size of the lesion is large enough to perform a biopsy and the location is approachable, we can readily differentiate a malignant lesion from a benign lesion, using a percutaneous needle biopsy (PCNB). However, if the size is too small to approach or the lesion is adjacent to a large vessel, it is sometimes difficult to perform PCNB for pathological confirmation. Especially, if the isolated mediastinal LNs were only found via a CT scan, we might consider mediastinoscopy to obtain adequate tissue for pathological confirmation. In the case of an isolated pulmonary nodule, if it is too small to obtain sufficient tissue, a surgical procedure, such as video-assisted thoracic surgery (VATS) or thoracotomy should be considered for pathological confirmation. In these difficult circumstances of tissue confirmation, an observation during a short-term follow-up may be considered.
Several studies have reported that positron-emission tomography (PET) is superior to conventional imaging, such as CT, in terms of detecting recurrences or metastases in patients with breast cancer or other types of cancer [4-7]. Therefore, PET could help physicians to differentiate malignant lesions from benign lesions in the case of a newly-detected isolated mediastinal LN or small pulmonary nodule during surveillance. However, PET is problematic since several benign hypermetabolic lesions, such as sarcoidosis or tuberculosis can mimic malignant lesions [8,9]. Therefore, PET alone cannot suffice as a definite diagnostic method to differentiate malignant lesions from benign lesions. In this difficult clinical situation, if there were clinical factors, which could facilitate the differentiation of malignancy from benignity, these would aid physicians in making the correct diagnosis.
In this study, we identified useful clinical factors that can be utilized in differentiating malignant lesions from benign lesions in breast cancer patients with a newly isolated mediastinal LN or small pulmonary nodule during surveillance.
Materials and Methods
1. Patients
We searched our database and located breast cancer patients who had undergone mediastinoscopy, VATS, and thoracotomy during surveillance after curative resection of breast cancer at Seoul National University Hospital between 1995 and 2008. All patients included in our study met the following criteria: histologically- or pathologically-proven breast cancer; underwent mastectomy or breast conserving surgery with intent to cure; received appropriate adjuvant treatment, including chemotherapy, radiotherapy, and hormone therapy; had a new isolated mediastinal LN or pulmonary nodule during surveillance; and underwent mediastinoscopy, VATS, or thoracotomy for a new, isolated mediastinal LN or pulmonary nodule for pathological confirmation. However, patients with concurrent metastases at other sites (i.e., liver or brain), even if they had a mediastinal LN or pulmonary nodule, were excluded.
We reviewed the medical records of patients for the following characteristics: age, pathologic stage (TNM) at diagnosis, hormone receptors status, HER2 status, the number and size of the lesion, and the value of maximal standardized uptake (mSUV) on PET. TNM stage was reclassified in accordance to American Joint Committe on Cancer (AJCC) 6th edition. Estrogen-receptor (ER) or progesterone-receptor (PR) positivity was defined as staining for ER or PR ≥ 10% of tumor cells. Breast cancer with no expression of both ER and PR was defined as a hormone receptor-negative breast cancer. HER2 positivity was defined as immunohistochemical analysis (IHC) of 3+ or fluorescence in situ hybridizationpositive if IHC of HER2 was 2+. The results of CT and PET were categorized into “metastasis” or “benign” as specified by radiologic impression. The above-mentioned clinical factors were retrospectively analyzed to identify clinical factors, which were useful to differentiate malignant lesions from benign lesions.
2. Pathologic diagnostic procedures
Tissue diagnostic procedures were conducted by mediastinoscopy, VATS, or thoracotomy, including lobectomy or wedge resection. The type of surgical procedure for tissue diagnosis was determined based on the location and extent of the lesions suspicious for malignancy.
3. 18F-Fluorodeoxyglucose acquisition
After eight hours of fasting, 5.18 MBq/kg (0.14 mCi/kg) of fluorodeoxyglucose was injected. Then, all patients rested for one hour. A whole-body 18F-fluorodeoxyglucose PET scan was performed from the skull base to the proximal thigh. For the whole-body emission scan, 9-bed positions were examined at 3 minutes per step. The obtained images were reconstructed onto a square matrix and corrected for attenuation. The region of interest was drawn around the metastatic lesion and mSUV was defined as the peak SUV value on the pixel within the region of interest. We measured and analyzed the highest SUV among the mSUVs of all lesions.
4. Statistical analyses
Among clinical factors, categorical variables were analyzed by chi-square or Fisher’s exact test. Continuous variables were evaluated using a Student’s t-test or Mann-Whitney U test. Comparisons with regard to the sensitivity and specificity were conducted using an exact McNemar’s test. The maximum joint sensitivity and specificity had a similar interpretation to the area under the receiver operating characteristic (ROC) curve, and its values range from 0.5 (no diagnostic value) to 1.0 (perfect test). A two-sided p-value < 0.05 was considered statistically significant. Clinical factors, which were proven to be crucial to differentiate a malignant lesion from a benign lesion, were chosen as risk factors. In addition, the risk of malignancy according to the number of risk factors was calculated using relative risk. Detection-free survival was defined as the overall median time from the initial breast cancer surgery to the first detection of mediastinal LN or small pulmonary nodule during surveillance. Detection-free survival was estimated using the Kaplan- Meier method. The log-rank test was used to compare detection- free survival between the groups. Statistics analyses were conducted using SPSS ver. 19.0 (SPSS Inc., Chicago, IL) and Stata ver. 11.2 (Stata Corp., College Station, TX).
5. Ethics
This study protocol was reviewed and approved by the Institutional Review Board of Seoul National University Hospital (IRB No. H-1204-055-405). The recommendations of the Declaration of Helsinki for biomedical research involving human subjects were also followed.
Results
1. Patients
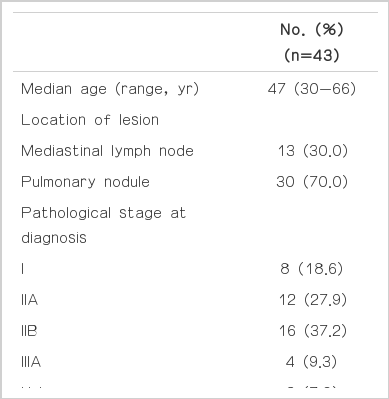
A total of 43 patients were enrolled. The median age was 47 years (range, 30 to 66 years). Thirteen patients (30%) had an isolated mediastinal LN and 30 (70%) had an isolated pulmonary nodule (Table 1). The initial stages at diagnosis (stage I, IIA, IIB, and IIIA) were 18.6%, 27.9%, 37.2%, and 9.3%, respectively. A total of 22 patients (51%) had ERand/ or PR-positive tumor, while 26 patients (60.5%) had HER2 negative breast cancer. Forty patients (93%) received adjuvant chemotherapy. Among the patients, 37% received anthracycline-based regimen, and 26% received both anthracycline and taxane-based regimen as adjuvant chemotherapy; 40% of the patients received tamoxifen or aromatase inhibitor as adjuvant hormonal therapy and 37% received adjuvant radiotherapy.
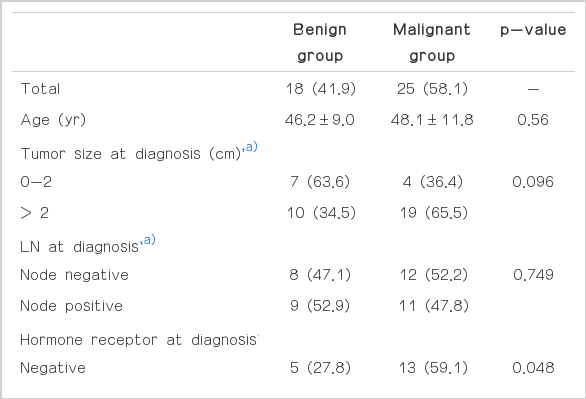
Among the 43 patients, 18 (41.9%) had pathologically confirmed benign lesions (benign group), and 25 (58.1%) had had pathologically confirmed malignant lesions (malignant group) (Table 2). Five of 13 patients (38.5%) who were presented with mediastinal LN had malignant lesion, and 20 of 30 patients (66.7%) who were presented with pulmonary nodule had malignant lesion. Among the benign lesions, chronic granulomatous inflammation (66.7%) was the most common benign lesion. Other benign lesions were nonneoplastic lung lesion (16.7%), sarcoidosis (5.6%), and harmartoma (5.6%). The median follow-up duration was 40.7 months (95% confidence interval [CI], 25.7 to 55.8 months).
2. Comparison of malignant and benign lesions
The comparison of the malignant group to the benign group, according to clinical factors, is presented in Table 3. The mean age, tumor size, and N stage at the time of diagnosis were shown to be similar between the two groups. The proportion of hormone-receptor negativity was significantly higher in the malignant group than in the benign group (59.1% vs. 40.9%, p=0.048). There was no significant difference between the two groups with regard to HER2 (p=0.688). The malignant group had a higher proportion of triple negativity than the benign group; however, the difference did not reach statistical significance (40.0% vs. 16.7%, p=0.113). The mean tumor size at the time of detection was larger in the malignant group than in the benign group, regardless of the suspicious lesion’s location (20.8 mm vs. 14.4 mm, p=0.024). However, the total number of suspicious lesions was not different between the two groups (2.2 vs. 2.8, p=0.361). PET was performed in 32 patients (74%). Malignant lesions were found to have a significantly higher mSUV than that of the benign lesions (6.4 vs. 3.4, p=0.021). A mSUV > 5.8 suggested that the lesion was likely to be malignant with a sensitivity of 53% and a specificity of 93% by ROC curve.
Table 4 presents the sensitivity and specificity of CT, PET, as well as combination of CT and PET. A combination of CT and PET exhibited greater sensitivity and specificity, compared with either one alone. However, no significant differences in the sensitivity and specificity were observed between CT and PET, CT and combination, as well as PET and the combination. The concordance rate with CT and pathology was 65%; the concordance rate was 59% with PET and pathology.
The overall detection-free survival was 24.8 months (95% CI, 14.2 to 35.4 months). The malignant group tended to have a longer detection-free survival than the benign group; however, the difference was not statistically significant (16.7 months vs. 34.8 months, p=0.386).
Among the 25 patients with metastatic mediastinal LN or small pulmonary nodule, 6 patients (24%) remain alive without any evidence of recurrence since the resection via mediastinoscopy, VATS, or thoracotomy. Among the 18 patients who were proven to have benign lesions, only one patient (5.6%) experienced recurrence in the bone and pleura after the resection of a benign lesion during surveillance.
3. Risk factor analysis
Hormone receptor negativity, large lesion size, and a high mSUV value were risk factors that favored malignant lesions. We analyzed the relative risk of malignancy in accordance to the number of risk factors (hormone receptor negativity, lesion size > median value [18 mm], and mSUV > median value) (Table 5). When patients were classified as specified by the number of risk factors, patients with 3 risk factors had a 2.7 times greater possibility of malignancy than those with 0 or 1 risk factor; the difference was statistically significant (95% CI, 1.417 to 5.020; p=0.025). Patients with 2 risk factors had a higher relative risk of malignancy, compared with patients with 0 or 1 risk factors; however, the difference did not achieve statistical significance (relative risk, 1.78; p=0.127). This finding suggests that the number of risk factors present can aid in the clarification of suspicious lesions.
Discussion
The aim of this study was to provide a useful risk modeling, which could differentiate malignant lesions from benign lesions in breast cancer patients with a newly-detected, isolated mediastinal LN or small pulmonary nodule during surveillance after curative resection. Our study demonstrates that hormone receptor status at diagnosis, tumor size, and mSUV of a suspicious lesion were useful clinical factors in predicting whether a new, isolated mediastinal LN or small pulmonary nodule was malignant.
A newly-detected, isolated mediastinal LNs or a small pulmonary nodule, which arises during surveillance, may present a diagnostic dilemma because further treatment and prognosis are dependent on whether the lesion is malignant. Therefore, tissue diagnosis is essential to differentiating malignant lesions from benign lesions. However, it is not always possible to perform tissue diagnosis. In the case of a small pulmonary nodule, it may be impossible to pathologically confirm if it is adjacent to the great vessels. If patients have an underlying lung disease, such as emphysema or large bullae, physicians cannot readily go forward with a tissue diagnosis due to the likeliness of severe complications.
A mediastinal LN is a rare site of metastasis in breast cancer. It is not easy to pathologically confirm since it is difficult to approach the lesion. A mediastinoscopic biopsy is the standard method of tissue attainment and pathological confirmation [10]. However, a mediastinoscopic biopsy is an invasive procedure, which requires general anesthesia. Elderly patients or patients with co-morbidity are likely to suffer complications if they undergo general anesthesia and mediastinoscopy. Furthermore, only a few studies on differentiation of malignant lesions from benign lesions in this setting have been done. Therefore, our study suggests clinical factors that may be useful to differentiating malignant lesions from benign lesions and provides the opportunity to avoid an invasive procedure. Recently, endoscopic ultrasound (EUS)- guided fine needle aspiration (FNA) has been introduced. One study reported that EUS-FNA had a high rate of pathological confirmation (91%) of a mediastinal LN with metastatic breast cancer [11]. Further study should be carried out in order to determine whether EUS-FNA should be an alternative method to achieving pathological confirmation of a mediastinal LN suspicious for malignancy in cancer patients.
Several non-invasive modalities have been actively studied to define suspicious lesions that arise during surveillance. PET is one of the useful imaging modalities to help physicians differentiate malignant lesions from benign lesions. For many tumors, PET achieves greater sensitivity and specificity than conventional imaging with regard to the detection of recurrent or metastatic disease [5]. Although the efficacy of PET for breast cancer differs among studies, meta-analysis and systemic review for the usefulness of PET in patients with breast cancer revealed good sensitivity (89-93%) and specificity (82-95%) for PET in detecting nodal or distant metastases [12,13]. The sensitivity and specificity of PET were significantly superior to those of CT. Several studies on additional analyses for LN or pulmonary lesion showed that the sensitivity and specificity of PET with regard to LNs were 80-97% and 91-100%, respectively and those for PET in regard to pulmonary nodules were 83% and 97%, respectively [14,15].
It is well-established that hormone-receptors are important prognostic factors for breast cancer. Several studies have reported that the ER–/PR– status was statistically significantly associated with an increased risk of distant and local recurrence [16-18]. Our study showed that patients with hormone receptor-negative breast cancer were more likely to have a malignant mediastinal LN or small pulmonary nodule than those with a hormone receptor-positive breast cancer. We also observed that isolated mediastinal LN or small pulmonary nodule in TN breast cancer had a tendency to be malignant, compared with those in non-TN breast cancer. These findings are consistent with the results of previous studies [19,20].
T stage and N stage at the time of diagnosis are important prognostic factors and are strongly associated with local recurrence and distant metastases [16-18,21-23]. However, in our study T stage and LN involvement at the time of diagnosis were not significant clinical factors, which helped differentiate malignant lesions from benign lesions. Although T stage at diagnosis was not associated with clinical factors for the prediction of a malignant lesion, new mediastinal LN or small pulmonary nodule in patients with large size of primary breast cancer (> 2 cm) at diagnosis was more likely to be malignant than those with small size of primary breast cancer (< 2 cm) at diagnosis. However, the difference was not statistically significant (p=0.096). Therefore, these findings should be further validated with future studies comprising a larger number of patients.
The distinct feature of our study is that we obtained adequate tissue for diagnosis from all patients by surgical procedures rather than aspiration or needle biopsy. One study was performed to evaluate the usefulness of PET in detecting mediastinal or internal mammary LN metastases and to determine the prevalence of suspicious lesions, as compared to conventional imaging modalities [24]. However, in the aforementioned study, pathological confirmation was conducted in only 4 out of 73 patients (5.5%) with suspicious lesions. Other patients were determined to have malignant or benign lesions by a follow-up CT imaging alone, without pathological confirmation. A malignant lesion was defined as a progressive enlargement of the suspicious lesion during a follow-up CT scan in that study. In contrast, all patients in our study underwent an appropriate surgical procedure to obtain adequate tissues. Thus, our study had a solid reference regarding the lesion characteristics for assessing the sensitivity or specificity of PET or even CT.
The limitation of our study is that it is retrospective in nature with a relatively small number of patients. However the paucity of studies focusing on this patient population and tissue confirmation in all enrolled patients add value to our study.
Conclusion
Our study suggests that the presence of hormone receptor, lesion size, and the mSUV value were useful clinical factors in differentiating between malignant and benign lesions for breast cancer patients with new mediastinal LN or small pulmonary nodule during surveillance. These findings could aid physicians in determining the likelihood of malignancy in suspicious lesions in the mediastinum; thus, reducing the need for an invasive procedure for pathological confirmation. Further validation study is warranted to confirm our findings.
Notes
Conflict of interest relevant to this article was not reported.