AbstractPurposeThe benefit of high-dose chemotherapy (HDC) for metastatic breast cancer (MBC) is controversial. We evaluated the efficacy and safety of HDC with cyclophosphamide, thiotepa, and carboplatin (CTCb) followed by autologous stem-cell transplantation (ASCT) for MBC patients.
Materials and MethodsFrom September 1994 to December 1999, 23 MBC patients were enrolled. All the patients received 2 to 10 cycles of induction chemotherapy. Before transplantation, 12 patients were in complete response (CR), nine were in partial response (PR), and two had progressive disease (PD). The HDC regimen consisted of cyclophosphamide 1,500 mg/m2/day, thiotepa 125 mg/m2/day and carboplatin 200 mg/m2/day intravenously for 4 consecutive days.
ResultsAfter ASCT, 13 patients (56%) had a CR, five (22%) had a PR, three (13%) had no change, while two (9%) showed a PD. Seventeen patients relapsed or progressed during the median follow-up of 78 months. The median progression-free survival (PFS) time was 11 months and the median overall survival (OS) time was 23 months. The 5-year PFS and OS rates were 22% and 25%, respectively. On the multivariate analyses, less than 4 involved lymph nodes was predictive of a better PFS and OS.
INTRODUCTIONMetastatic breast cancer (MBC) is usually regarded as an incurable disease having a median survival time of approximately 2 years after the diagnosis of metastases (1). In such patients, there are not many complete responses (CR) with conventional-dose chemotherapy.
Retrospective studies have indicated a positive correlation between increasing the dose intensity and improving the response rates for MBC (2). In this respect, autologous stem-cell transplantation (ASCT) allows for dose escalation beyond the normal bone marrow tolerance, which is needed to overcome the resistance of tumor cells to chemo and radiation therapy so as to produce better outcomes. In the 1980s and 1990s, several phase II studies reported promising results for MBC after high-dose chemotherapy (HDC) with ASCT (3~5), and this has prompted the number of HDC studies to increase. However, the randomized studies reported to date have failed to show any clear advantage of the high-dose approach. The overall survival (OS) has been similar for HDC and the conventional-dose chemotherapy, while progression-free survival (PFS) was improved in the HDC group (6~14).
In 1998, we reported the preliminary result of phase II study of HDC with cyclophosphamide, thiotepa, and carboplatin (CTCb) followed by ASCT for 11 patients with metastatic or high-risk breast cancer (15). This study was closed down in July 2000 due to the lack of evidence from randomized studies demonstrating any survival benefit for HDC (10). Here in this study, we report the updated outcome of a phase II study on 23 metastatic patients after a median follow-up of 78 months and a maximum follow-up of 10 years.
MATERIALS AND METHODS1) PatientsFemale patients were considered eligible if they had histologically proven MBC; these patients were from 16 to 65 years of age, they had an Eastern Cooperative Oncology Group performance status from 0 to 2 and they had adequate organ function (defined as leukocytes ≥4,000/µL, platelets ≥100,000/µL, transaminases ≤2 times the upper normal limit, total bilirubin ≤2.0 mg/dL, serum creatinine ≤2.0 mg/dL and a cardiac ejection fraction ≥50%). The study was approved by our Institutional Review Board in accordance with the Helsinki Declaration, and a written informed consent was obtained from each patient.
Most patients underwent mastectomy or a breast-conserving operation and all patients received 2 to 10 cycles (median number of cycles: 4) of induction chemotherapy. CAF (cyclophosphamide 600 mg/m2, doxorubicin 60 mg/m2 and 5-fluorouracil 600 mg/m2 every 3 weeks) was the preferred regimen and the remaining patients received TP (paclitaxel 135 mg/m2 and cisplatin 75 mg every 3 weeks) after previously having received anthracycline-containing chemotherapy for metastatic disease. Local therapy consisting of radiotherapy or surgery was allowed, and patients rendered free of measurable disease by surgery were categorized as having achieved a CR. For patients with bone-only metastases, a partial response (PR) was defined as the resolution of bone pain and a reduction or stabilization in the size and/or number of bony lesions on radionuclide scans following induction chemotherapy.
2) TreatmentPeripheral-blood stem cells mobilization was induced by chemotherapy plus growth factor (G-CSF, 10 µg/kg/day subcutaneously). Each patient was administered cyclophosphamide (4 g/m2), CAF or TP. The peripheral-blood stem cells were collected by leukapheresis until at least 3×106 CD34+ cells/kg were harvested (16).
The HDC regimen consisted of cyclophosphamide 1,500 mg/m2/day, thiotepa 125 mg/m2/day and carboplatin 200 mg/m2/day intravenously on days -7 to -4 (5). G-CSF was begun the day after stem cell infusion and it was continued until the absolute neutrophil count reached 1,000/µL or more on 2 consecutive days.
All the patients received prophylactic antimicrobials/antivirals consisting of oral ciprofloxacin and acyclovir. The patients were transfused with irradiated red blood cells and platelets to maintain their hemoglobin levels at greater than 8 g/dL and their platelet counts at greater than 20,000/µL.
The standard response criteria were used (17), and the evaluation of toxicity was performed according to National Cancer Institute-Common Toxicity Criteria (NCI-CTC).
3) Statistical AnalysisThe PFS and OS were estimated using the Kaplan-Meier method and they were calculated from the date of stem cell infusion. PFS was measured until the date of disease progression or death from any cause, and OS was measured until the date of death from any cause. For patients who did not experience these events, their data were censored at the time of their last follow-up visit. The log-rank test was used to compare survival times between the patient subgroups, and the Cox proportional hazards model was used to estimate hazard ratios and to perform regression analysis. p values of 0.05 or less were considered significant.
RESULTS1) Patient Characteristics and TreatmentFrom September 1994 to December 1999, a total of 23 women were enrolled. The patient characteristics are summarized in Table 1. Among them, 20 patients had recurrent disease and three presented with metastatic disease. Forty-eight percent of the patients had more than one site of metastatic disease and 61% had visceral disease. The induction chemotherapy was CAF in 13 patients (57%) and TP in 10 patients who had previously received CAF for metastatic disease. After the induction chemotherapy, 12 patients were in CR, nine were in PR and two showed progressive disease (PD).
A median of four sessions (range: 2 to 14 sessions) was required to obtain at least 3×106 CD34+ cells/kg for transplantation (median yield: 5.4×106; range: 1.2×106 to 19.9×106). In six patients (26%), less than the required target number of cells was harvested. The median time to neutrophil engraftment (≥500/µL) was 11 days (range: 8 to 35 days), and the median time to independence from platelet transfusion (≥20,000/µL) was 15 days (range: 9 to 31 days). There were five cases of platelet engraftment failure, and one patient died of hemorrhage nine months after ASCT. The median in-patient length of stay after stem-cell infusion was 22 days (range: 14 to 130 days).
All the patients became transfusion-dependent and twenty of them developed an episode of neutropenic fever that required intravenous antibiotics; however, no documented infection was noted. The most common grade 3 or 4 nonhematologic toxicity was diarrhea that occurred in six patients (26%). Other grade 3 nonhematologic toxicities were mucositis in one, nausea/vomiting in three and azotemia in two. There was one case of hemorrhagic cystitis despite the concomitant administration of mesna.
2) Initial response to ASCTThe 12 patients in CR before transplantation maintained their CR after ASCT. Among the 9 patients who entered ASCT with PR, one (11%) converted to CR, five (56%) achieved a better PR, and one had no change, while two showed a PD. Two patients (9%) who showed a PD after induction chemotherapy had no change in status (Table 2).
3) SurvivalWith a median follow-up of 78 months from the time of ASCT (range: 55 to 119), 17 patients relapsed or progressed. In total, nine patients showed progression at the sites of their previous disease, one showed progression at a new site, and seven showed progression at both the previous sites and new sites. The median duration of PFS was 11 months [95% confidence interval (CI): 6 to 15 months] and the median duration of OS was 23 months (95% CI: 0 to 56 months) (Fig. 1). The 5-year PFS and OS rates were 22±9% and 25±9%, respectively.
Univariate analysis was performed to determine the factors that were predictive of PFS and OS (Table 3). The PFS was found to be significantly superior for patients who had less than 4 involved lymph nodes, patients with the absence of liver metastases and if the patients had a CR to induction chemotherapy (Fig. 2). The induction regimen was a marginally significant factor for PFS (p=0.064). A shorter disease-free interval, less than 4 involved lymph nodes, a single metastatic site, the absence of liver metastases, an induction regimen with CAF and a CR to induction chemotherapy were factors predictive of a better OS. The significant factors on the multivariate model for survival are shown in Table 4. The number of involved lymph nodes and the response to induction chemotherapy remained significant for PFS, and the disease-free interval and the number of involved lymph nodes were significant for OS. Patients with less than 4 involved lymph nodes showed higher 5-year PFS and OS rates of 42% vs. 0%, respectively. The hazard ratios for disease progression and death were 3.4 (95% CI, 1.1 to 10.4; p=0.036) and 8.3 (95% CI, 1.7 to 40.8; p=0.009), respectively.
DISCUSSIONHDC of cyclophosphamide, thiotepa, and carboplatin (CTCb) followed by ASCT for patients with MBC had an acceptable toxicity; however, this treatment did not show a survival benefit. After a median follow-up of 78 months from the date of stem-cell infusion, the 5-year PFS and OS rates were 22% and 25%, respectively.
For MBC patients who were transplanted after their response to first-line chemotherapy, not only did HDC produce the highest response and CR rate ever reported using conventional-dose chemotherapy, but this treatment also achieved long-term PFS rates of 10% to 25% (7). On the other hand, a retrospective study has suggested that these promising results could be better explained by a bias in patient selection (a younger age and better performance status), extensive tumor staging and the selection requirement of chemosensitivity (18).
Eight randomized trials on the role of HDC for MBC patients have been published to date, but only seven of these are evaluable because the Bezwoda trial (19) was disaccredited (Table 5). Moreover, the OS has been evaluated in only four of seven studies since three studies (6,9,14) had a crossover design. In the four evaluable trials, the OS was similar for the HDC groups and the conventional-dose chemotherapy groups, while PFS was improved for the HDC group in six of seven trials, with the only exception being the Philadelphia study (10). In the Philadelphia PBT-1 study (10,11), an intent-to-treat analysis showed no difference in the PFS or OS rates between the transplantation and conventional-dose chemotherapy groups. However, that study was limited by a 45% drop-out rate (34% before and 11% after randomization), and only 45 patients having a CR were treated in study: this conferred only a 20% power to detect a 20% absolute OS difference in the subgroups that may benefit most from HDC (7,20). Moreover, the conventional-dose chemotherapy was continued until disease progression or for up to 24 cycles, so this cannot be considered as conventional treatment.
The delay in disease relapse or the progression of metastatic disease itself is clinically important. Additionally, since none of the reported studies randomized more than 220 patients, their statistical power may have been too limited to detect any meaningful survival differences. In the French FEGASE-04 trial (8), there appeared to be large difference in favor of the transplant group for the PFS (median: 35 vs. 20 months) and OS (median: 43 vs. 20 months; 5-year OS rates: 30% vs. 18%), and none of the results reached statistical significance due to the very small sample size (n=61). A longer follow-up period is also needed to see whether the PFS advantage translates into an OS benefit. Berry et al. (21) retrospectively compared the survival of 635 MBC patients who were enrolled in the Cancer and Leukemia Group B (CALGB) trials of standard-dose chemotherapy with the survival of 441 HDC-treated MBC patients from the Autologous Blood and Marrow Transplant Registry (ABMTR). Patient survival was similar for the first 2 years, but by 3 years, there was a statistically significant advantage for the HDC of 39% vs. 31%. Meanwhile, it is possible that the benefit from HDC is restricted to certain subgroups of patients, resulting in relatively small difference for the whole study group. In the retrospective studies, liver involvement, prior adjuvant chemotherapy, a shorter disease-free interval and negative estrogen receptor status were poor prognostic factors for both PFS and OS (22,23).
Our median PFS and OS of 11 and 25 months, respectively, are consistent with other HDC trials [weighted average PFS: 10 months (range: 6 to 20 months); weighted average OS: 19 months (range: 10 to 24 months)] (5,6,9~13,24). Furthermore, we did not restrict patients to those responding to initial induction chemotherapy as in many of the HDC trials for MBC. Two of our patients did not achieve a response to induction chemotherapy and 10 (43%) of our patients received 2 or more different regimens for metastatic disease. Despite this heterogeneity, the CR rate after ASCT was 56%. This CR rate was higher than those CR rates seen with conventional-dose chemotherapy (weighted average: 17%; range: 4% to 23%) and it was comparable with other HDC trials for MBC patients (weighted average: 40%; range: 6% to 55%) (3~5,24). However, of the nine patients who received HDC after they achieved a PR to induction chemotherapy, only one patient (11%) went on to achieve a CR with HDC. This low PR to CR conversion rate in our study was different from the results in the vast majority of phase II HDC trials, in which PR to CR conversion rates of 20% to 60% were reported (4,5,7). The conflicting results may derive from differences in the design of the trials, the drug regimens that were used and the patients that were studied. About half of our patients were heavily treated with more than one regimen prior to HDC, and so the tumor cells that had already survived the standard regimens were more likely to be resistant at the time of reinduction and HDC.
Cox multivariate analysis demonstrated that the number of involved lymph nodes was an independent predictor of both PFS and OS. Additionally, the patients achieving a CR to induction chemotherapy showed significantly higher 5-year PFS rate than those patients who were not in CR prior to their ASCT (33% vs. 9%). Other authors have reported similar results (5,10,22,24), and interestingly, the induction regimen with CAF showed a tendency for a better PFS and OS. All the patients whose induction regimens were TP received this regimen as a second- or third-line chemotherapy after prior use of CAF for metastatic disease, while the remaining patients received CAF as first-line treatment (except for one patient). A possible explanation is that those patients whose tumors have not yet failed to respond to the best treatment regimen would be the most likely to benefit from HDC. However, because this study was small and it was not a true population-based study, any survival analysis must be interpreted very cautiously.
In the present study, most nonhematologic toxicities were mild and tolerable, and one patient died of hemorrhage nine months after ASCT due to lack of platelet engraftment. There were five cases of platelet engraftment failure, and in four of these, less than the targeted number of cells was harvested. Three of the four patients who received surgery for metastatic disease remained disease-free, although 84% of the remaining patients relapsed or progressed at the sites of their previous disease or at both previous and new sites. The benefit for surgical resection of distant metastases is still a matter of debate despite some encouraging reports (25).
CONCLUSIONSThis is the only reported series with a prolonged follow-up period (median: 78 months, and maximum: 10 years) in Korea with regards to HDC for MBC. HDC is not currently accepted as a standard therapy in patients with MBC. However, the patients in this study had a 5-year PFS rate of 22% and a 5-year OS rate of 25% after HDC with ASCT.
References1. Mick R, Begg CB, Antman KH, Korzun AH, Frei E 3rd. Diverse prognosis in metastatic breast cancer: Who should be offered alternative initial therapies? Breast Cancer Treat Res. 1989;13:33–38.
2. Hryniuk W, Bush H. The importance of dose intensity in chemotherapy of metastatic breast cancer. J Clin Oncol. 1984;2:1281–1288. PMID: 6387060
3. Peters WP, Shpall EJ, Jones RB, Olsen GA, Bast RC, Gockerman JP, et al. High-dose combination alkylating agents with bone marrow support as initial treatment for metastatic breast cancer. J Clin Oncol. 1988;6:1368–1376. PMID: 3047332
4. Kennedy MJ, Beveridge RA, Rowley SD, Gordon GB, Abeloff MD, Davidson NE. High-dose chemotherapy with reinfusion of purged autologous bone marrow following dose-intense induction as initial therapy for metastatic breast cancer. J Natl Cancer Inst. 1991;83:920–926. PMID: 1906111
5. Antman K, Ayash L, Elias A, Wheeler C, Hunt M, Eder JP, et al. A phase ii study of high-dose cyclophosphamide, thiotepa, and carboplatin with autologous marrow support in women with measurable advanced breast cancer responding to standard-dose therapy. J Clin Oncol. 1992;10:102–110. PMID: 1727912
6. Peters WP, Jones RB, Vredenburgh J, Shpall EJ, Hussein A, Elkordy M, et al. A large, prospective, randomized trial of high-dose combination alkylating agents (CPB) with autologous cellular support (ABMS) as consolidation for patients with metastatic breast cancer achieving complete remission after intensive doxorubicin-based induction therapy (AFM). Proc Am Soc Clin Oncol. 1996;16:121(abstr 149)
7. Nieto Y. The verdict is not in yet. Analysis of the randomized trials of high-dose chemotherapy for breast cancer. Haematologica. 2003;88:201–211. PMID: 12604410
8. Lotz J, Cure H, Janvier M, Morvan F, Asselain B, Guillemot M, et al. High-dose chemotherapy (HD-CT) with hematopoietic stem cells transplantation (HSCT) for metastatic breast cancer (MBC): results of the French protocol PEGASE 04. Proc Am Soc Clin Oncol. 1999;18:43a(abstr 161)
9. Madan B, Broadwater G, Rubin P, Edwards J, Long G, Chao NC, et al. Improved survival with consolidation high-dose cyclophosphamide, cisplatin and carmustine (HD-CPB) compared with observation in women with metastatic breast cancer (MBC) and only bone metastases treated with induction adriamycin, 5-fluorouracil and methotrexate (AFM): A phase III prospective randomized comparative trial. Proc Am Soc Clin Oncol. 2000;19:48a(abstr 184)
10. Stadtmauer EA, O'Neill A, Goldstein LJ, Crilley PA, Mangan KF, Ingle JN, et al. The Philadelphia Bone Marrow Transplant GroupConventional-dose chemotherapy compared with high-dose chemotherapy plus autologous hematopoietic stem-cell transplantation for metastatic breast cancer. N Engl J Med. 2000;342:1069–1076. PMID: 10760307
11. Stadtmauer EA, O'Neill A, Goldstein LJ, Crilley PA, Mangan KF, Ingle JN, et al. Conventional-dose chemotherapy compared with high-dose chemotherapy (HDC) plus autologous stem-cell transplantation (SCT) for metastatic breast cancer: 5-year update of the 'Philadelphia Trial' (PBT-1). Proc Am Soc Clin Oncol. 2002;21:43a(abstr 169)
12. Crump M, Gluck S, Stewart D, Levine M, Pritchard K, Kirkbride P, et al. A randomized trial of high-dose chemotherapy (HDC) with autologous peripheral blood stem cell support (ASCT) compared to standard therapy in women with metastatic breast cancer: a National Cancer Institute of Canada (NCIC) clinical trial group study. Proc Am Soc Clin Oncol. 2001;20:21a(abstr 82)
13. Biron P, Durand M, Roche H, Delozier T, Battista C, Fargeot P, et al. High dose thiotepa (TTP), cyclophosphamide (CPM) and stem cell transplantation after 4 FEC 100 compared with 4 FEC alone allowed a better disease free survival but the same overall survival in first line chemotherapy for metastatic breast cancer. Results of the PEGASE 03 French Protocol. Proc Am Soc Clin Oncol. 2002;21:42aabstr 167
14. Schmid P, Samonigg H, Nitsch T, Huebner G, Kreienberg R, Schultze W, et al. Randomized trial of up front tandem high-dose chemotherapy (HD) compared to standard chemotherapy with doxorubicin and paclitaxel (AT) in metastatic breast cancer (MBC). Proc Am Soc Clin Oncol. 2002;21:43a(abstr 171)
15. Cho SH, Kim SW, Min YJ, Choi SJ, Kim JK, Kim TW, et al. High dose cyclophosphamide, thiotepa, and carboplatin followed by autologous peripheral stem cell rescue in patients with responsive metastatic or high-risk primary breast cancer. J Korean Cancer Assoc. 1998;30:100–105.
16. Suh C, Kim HJ, Kim SH, Kim S, Lee SJ, Lee YS, et al. Low-dose lenograstim to enhance engraftment after autologous stem cell transplantation: a prospective randomized evaluation of two different fixed doses. Transfusion. 2004;44:533–538. PMID: 15043569
17. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. PMID: 2358840
18. Rahman ZU, Frye DK, Buzdar AU, Smith TL, Asmar L, Champlin RE, et al. Impact of selection process on response rate and long-term survival of potential high-dose chemotherapy candidates treated with standard-dose doxorubicin-containing chemotherapy in patients with metastatic breast cancer. J Clin Oncol. 1997;15:3171–3177. PMID: 9336352
19. Bezwoda WR, Seymour L, Dansey RD. High-dose chemotherapy with hematopoietic rescue as primary treatment for metastatic breast cancer: a randomized trial. J Clin Oncol. 1995;13:2483–2489. PMID: 7595697
20. Livingston R, Crowley J. Commentary on PBT-1 study of high-dose consolidation versus standard therapy in metastatic breast cancer. J Clin Oncol. 1999;17(supple 11):22–24. PMID: 10630257
21. Berry DA, Broadwater G, Klein JP, Antman K, Aisner J, Bitran J, et al. High-dose versus standard chemotherapy in metastatic breast cancer: comparison of cancer and leukemia group b trials with data from the autologous blood and marrow transplant registry. J Clin Oncol. 2002;20:743–750. PMID: 11821456
22. Rowlings PA, Williams SF, Antman KH, Fields KK, Fay JW, Reed E, et al. Factors correlated with progression-free survival after high-dose chemotherapy and hematopoietic stem cell transplantation for metastatic breast cancer. JAMA. 1999;282:1335–1343. PMID: 10527180
23. Rizzieri DA, Vredenburgh JJ, Jones R, Ross M, Shpall EJ, Hussein A, et al. Prognostic and predictive factors for patients with metastatic breast cancer undergoing aggressive induction therapy followed by high-dose chemotherapy with autologous stem-cell support. J Clin Oncol. 1999;17:3064–3074. PMID: 10506601
24. Damon LE, Wolf JL, Rugo HS, Gold E, Zander AR, Cassidy M, et al. High-dose chemotherapy (CTM) for breast cancer. Bone Marrow Transplant. 2000;26:257–268. PMID: 10967563
25. Bathe OF, Kaklamanos IG, Moffat FL, Boggs J, Franceschi D, Livingstone AS. Metastasectomy as a cytoreductive strategy for treatment of isolated pulmonary and hepatic metastases from breast cancer. Surg Oncol. 1999;8:35–42. PMID: 10885392
Fig. 1(A) Progression-free survival and (B) overall survival (median: 11 and 23 months, respectively). 
Fig. 2Progression-free survival according to (A) the number of positive nodes (p=0.03) (B) the presence of liver metastases (p=0.028) and (C) the response to induction chemotherapy (p=0.0089). 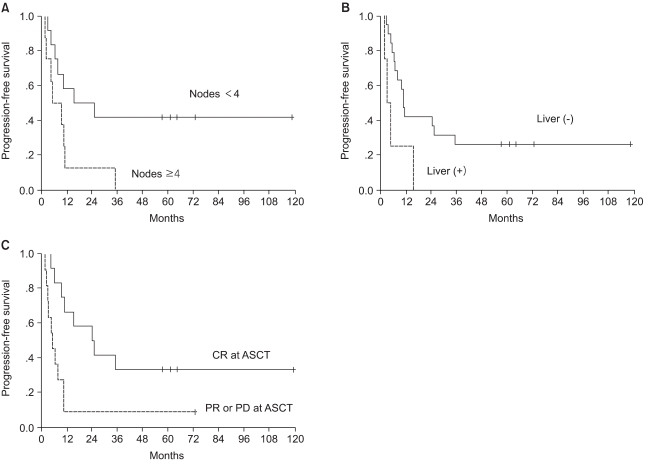
|
|