AbstractPurposeThe purpose of this study was to determine whether chemoradiation (CCR) is efficient for improving prognosis, compared with systemic chemotherapy (SC), in patients with stage IVB cervical cancer who have distant lymphatic metastasis.
Materials and MethodsAmong 2,322 patients with cervical cancer between January 2000 and March 2010, 43 patients (1.9%) had stage IVB disease. After exclusion of 19 patients due to insufficient data and hematogenous metastasis, 24 patients (1%) who received CCR (n=10) or SC (n=14) were enrolled. We compared tumor response, progression-free survival (PFS) and overall survival (OS), and disease recurrence between CCR and SC.
ResultsComplete response rates were 60% and 0% after CCR and SC (p<0.01). Grade 3 or 4 leukopenia was more common in patients treated with CCR (24.4% vs. 9.1%, p=0.03), whereas grade 3 or 4 neuropenia was more frequent in those treated with SC (28.4% vs. 11.1%, p=0.03). Development of grade 3 proctitis occurred as a late radiotherapy (RT)-related toxicity in only one patient (10%) treated with CCR. In addition, squamous cell carcinoma and CCR were favorable prognostic factors for improvement of PFS (adjusted hazard ratios [HRs], 0.17 and 0.12; 95% confidence intervals [CIs], 0.04 to 0.80 and 0.03 to 0.61), and only CCR was significant for improvement of OS (adjusted HR, 0.15; 95% CI, 0.02 to 0.90). However, no differences in the rate and pattern of disease recurrence were observed between CCR and SC.
IntroductionDisease status at diagnosis is crucial to selection of methods for treatment of cervical cancer. In early-stage cervical cancer, especially International Federation of Gynaecology and Obstetrics (FIGO) stage I to IIA disease, surgery is a primary standard treatment, and radiotherapy (RT) or concurrent chemoradiation (CCR) using platinum is also recommended, whereas CCR is recommended as the optimal treatment for locally advanced cervical cancer (stage IB2 to IVA disease) [1]. On the other hand, systemic chemotherapy (SC) is known as the only treatment for recurrent or stage IVB cervical cancer showing dissemination of cancer cells to distant organs [2]. However, SC has a limited role in prolonging survival, and has a low response rate of 20-30% [3]. Thus, it is only recommended for patients with distant metastasis who are not candidates for loco-regional treatment using RT or exenterative surgery [4].
To date, in relevant clinical trials, stage IVB and recurrent cervical cancers have been considered to be similar due to lack of enrolled patients with stage IVB cervical cancer (approximately 2.6%) [5-7]. In recurrent cervical cancer where the patient received prior chemotherapy, development of chemo-resistance may occur through several changes in drug transport, leading to reduced intracellular accumulation, and activated drug detoxification by elevated levels of intracellular scavengers, including glutathione and apoptotic cell death pathways [8], and prior ionizing radiation can also be a cause of resistance to RT [9]; therefore, it should be regarded as having different biologic characteristics when compared with stage IVB cervical cancer, suggesting that the combination of these two groups may not be appropriate in clinical trials. Nevertheless, due to the rarity, few studies for treatment of patients with stage IVB cervical cancer only have been reported [6,10,11]. Thus, the purpose of the current study was to evaluate the question of whether CCR is efficient for improving prognosis by comparing the efficacy and toxicity between CCR and SC in patients with stage IVB cervical cancer.
Materials and Methods1. PatientsThe material for the current study was derived from a database of 2,322 patients with cervical cancer in our institute between January 2000 and March 2010. Approval by the Institutional Review Board of Seoul National University Hospital was obtained in advance, and informed consent was waived because of a retrospective design using medical record review.
Stage IVB disease was defined as a primary cervical cancer with distant lymphatic spread beyond the abdomen. The distant spread of tumor was confirmed by imaging studies, such as computed tomography (CT), positron emission tomography (PET), or PET-CT, and fine needle aspiration biopsy on distant metastatic lesions. However, we excluded patients with recurrent cervical cancer, only para-aortic lymph node metastasis within the abdomen, and hematogenous metastasis. In addition, those with insufficient clinicopathologic data, including age, histology, disseminated pattern, number of distant metastatic sites, methods of primary treatment, chemotherapeutic regimen, and cycles of chemotherapy were not enrolled.
2. Treatment methodsFor CCR, external-beam radiotherapy (EBRT) was delivered to the whole pelvis with 6 or 10 megavoltage photons through parallel-opposed (antero-posterior/postero-anterior) ports or the four-field box technique. The upper and lower borders of the EBRT ports were at the L5-S1 junction and at least 2 cm below the gross tumor of the cervix. The lateral edges were set to 1.5 cm lateral to the bony pelvis. The anterior and posterior borders were the anterior aspect of the symphysis pubis and the S2-S3 junction, respectively. The standard dose was 50.4 Gy, consisting of 1.8-2 Gy fractions once daily for five days per week.
Depending on the decision by radiation oncologists in our institute, additional radiation was applied to the parametrium, para-aortic lymph nodes, and supraclavicular lymph nodes. The radiation dose to the bilateral parametrium was boosted to 60.4 Gy with a 4-cm wide midline shielding, and the field of irradiation with 45-61.2 Gy to the para-aortic lymph nodes was extended with EBRT to the suspicious lesions over the T12-L1 junction. In addition, the radiation dose to supraclavicular lymph nodes was delivered to 61.2-70.2 Gy. For brachytherapy, intra-cavitary irradiation was delivered by a Fletcher-Suit unit with a 137Cs source (33.5-35 Gy).
Concurrent chemotherapy consisted of three cycles of paclitaxel (135 mg/m2)/carboplatin (area under the curve [AUC] 5, Calvert's formula) every three weeks, two cycles of 5-fluorouracil (1,000 mg/m2 for five consecutive days)/cisplatin (60 mg/m2 on the first day) every four weeks, or two cycles of cisplatin (70 mg/m2) or carboplatin (AUC 5, Calvert's formula) alone every four weeks. After CCR, three to six additional cycles of adjuvant chemotherapy were administered. For SC, paclitaxel (175 mg/m2)/carboplatin (AUC 5, Calvert's formula) every three weeks, 5-fluorouracil (1,000 mg/m2 for five consecutive days)/cisplatin (60 mg/m2 on the first day) every three weeks, or cisplatin (70 mg/m2) or carboplatin (AUC 5, Calvert's formula) alone every three weeks was administered for three to 12 cycles.
3. ObjectivesThe primary objective was to compare tumor response and toxicity between CCR and SC. Tumor response was assessed using physical examination and imaging studies according to the response evaluation in solid tumors (RECIST) criteria four weeks after completion of primary treatment [12]. Complete response (CR) was defined as the disappearance of all lesions for at least four weeks, and partial response (PR) and progressive disease (PD) were defined as a reduction of more than 30% and an increase of more than 20% in the sum of the perpendicular diameters of lesions, respectively. The other disease status was defined as stable disease (SD). In addition, acute hematological and late RT-related toxicities were compared between the two treatments according to the Common Terminology Criteria for Adverse Events version 3.0 (CTCAE) [13].
The secondary objective was to compare progression-free survival (PFS) and overall survival (OS) between CCR and SC. PFS was defined as the time elapsed from the date of diagnosis to the date of clinically-proven recurrence. OS was calculated as the length of time from the date of diagnosis to the date of cancer-related death or the end of the study. In addition, favorable prognostic factors for improvement of survival were evaluated in patients with stage IVB cervical cancer. Thereafter, we compared the rate and pattern of disease recurrence between the two treatments.
4. Statistical analysisStatistical analysis was performed using SPSS ver. 19.0 (SPSS Inc., Chicago, IL). Clinico-pathologic characteristics between patients who received CCR and those who received SC were compared using the Mann-Whitney U-test and χ2 or Fisher's exact test. In addition, we performed survival analysis using the Kaplan-Meier method with the log-rank or Breslow test, and Cox's proportional hazard regression model using hazard ratio (HR) and 95% confidence interval (CI) for determination of favorable prognostic factors. A p<0.05 was considered statistically significant.
Results1. Patients' characteristicsAmong a total of 2,322 patients with cervical cancer, 2,292 were excluded for the following reasons: stage I (n=1,489), stage II (n=682), stage III (n=67), stage IVA (n=37), insufficient data (n=17), and hematogenous metastasis (n=6). Thus, the remaining 24 patients (1%), including 14 patients (58.3%) treated with SC and 10 patients (41.7%) treated with CCR were enrolled. The clinico-pathologic data for all patients are shown in Table 1. The median age was 50 years (range, 36 to 83 years), and the median duration of follow-up was 20.3 months (range, 1.8 to 83.8 months). Histologically, 21 patients (87.5%) were diagnosed with squamous cell carcinoma (SCCA), two (8.3%) had poorly differentiated carcinoma, and one (4.2%) had adenocarcinoma.
A summary of targeted areas for CCR is shown in Table 2. Among the 10 patients who received EBRT to the whole pelvis, additional irradiation was applied to seven patients (70%) with para-aortic lymph node (LN), five patients (50%) with a primary cervical mass using brachytherapy, three patients (30%) with parametrium, and three patients (30%) with supraclavicular LN. In addition, chemotherapy using paclitaxel/carboplatin was administered more frequently in patients who received CCR than in those who received SC (70% vs. 28.6%, p=0.04).
2. Response and toxicityAmong 10 patients treated with CCR, six (60%) and two (20%) had CR and PR, whereas two (20%) had SD. Among 14 patients treated with SC, there were no patients with CR. Among them, only seven (50%) patients had PR, whereas two (14.3%) and five (35.7%) had SD and PD. Although no difference was observed in the overall response rate (80% vs. 50%, p=0.21), the CR rate was higher in patients treated with CCR (60% vs. 0%, p<0.01).
Ten patients treated with CCR received 45 cycles while 14 patients treated with SC received 88 cycles of chemotherapy. When we compared acute hematological and late RT-related toxicities between the two treatments, grade 3 or 4 leukopenia was more common in patients treated with CCR (24.4% vs. 9.1%, p=0.03), whereas grade 3 or 4 neutropenia was more frequent in those treated with SC (28.4% vs. 11.1%, p=0.03). However, no differences in grade 3 or 4 anemia and thrombocytopenia were observed between the two treatments. In addition, one patient (10%) who underwent brachytherapy as well as previous EBRT developed grade 3 RT-related proctitis within one month after CCR (Table 3). Finally, conservative treatment for two weeks resulted in resolution of proctitis.
3. Survival and disease recurrenceCCR, SCCA, and CR after the primary treatment showed an association with better PFS and OS than SC, non-SCCA, and non-CR after the primary treatment (Fig. 1). However, there were no differences in PFS and OS based on the chemotherapeutic regimen and cycles of chemotherapy (data not shown). In addition, SCCA and CCR were favorable prognostic factors for improvement of PFS (adjusted HRs, 0.17 and 0.12; 95% CIs, 0.04 to 0.80 and 0.03 to 0.61), and only CCR was significant for improvement of OS (adjusted HR, 0.15; 95% CI, 0.02 to 0.90) (Table 4).
Eighteen patients (75%) showed disease recurrence. Among them, six (60%) and 12 (50%) patients were treated with CCR and SC, suggesting no difference in the disease recurrence rate (p=0.72). In addition, no differences in the site and pattern of disease recurrence were observed between the two treatments (Table 5).
DiscussionIn the current study, we attempted to determine whether CCR could improve clinical outcomes in patients with stage IVB cervical cancer when compared with SC, and the following three meaningful results were obtained. First, CCR increased CR after the primary treatment when compared with SC (60% vs. 0%). The reason is that RT contributes to the dramatic decrease of tumor burden, which cannot be controlled by SC if RT is applied to most targeted lesions. Results of the current study showed that grade 3 or 4 leukopenia was more common, and grade 3 RT-related toxicity was 10% in patients treated with CCR, therefore, we should be cautious in the increase of toxicity of SC by RT when we consider CCR for treatment of cervical cancer.
Second, CCR was efficient for improvement of PFS and OS (adjusted HRs, 0.12 and 0.15; 95% CIs, 0.03 to 0.61 and 0.02 to 0.90), suggesting the possibility that CCR may be superior to SC for patients with stage IVB cervical cancer who have distant lymphatic metastasis. Although the reason is not clear, previous studies have emphasized that because RT shows a better prognosis in patients with distant lymphatic metastasis, the area of RT should be extensive enough to include distant lymphatic spread of cancer cells in advanced or recurrent esophageal and cervical cancers [14,15]. These findings support the potential feasibility of CCR for treatment of stage IVB cervical cancer with distant lymphatic metastasis [16]. In addition, because cervical cancer tends to spread primarily by direct extension to the adjacent organs and via lymphatics, tumor volume-directed RT could be applied with the appropriate radiation dose in patients with distant lymphatic spread [17]. However, due to the wide range of the radiation field and a limitation of the total dose for protecting the function of metastatic organs by increased toxicity in stage IVB cervical cancer [18], which excluded patients with stage IVB cervical cancer who had hematogenous metastasis, patients with hematogenous metastasis are limited to RT. In the current study, although the preferred regimen differed between CCR (paclitaxel/carboplatin, 70%) and SC (5-fluorouracil/cisplatin, 57.2%), there was no difference in survival based on the chemotherapeutic regimen. In addition, previous studies have reported similar tumor response to paclitaxel/carboplatin and 5-fluorouracil/cisplatin in patients with metastatic cervical cancer (45-47% vs. 47-48%) [19].
Third, SCCA was a prognostic factor for better PFS in patients with stage IVB cervical cancer (adjusted HR, 0.17; 95% CI, 0.04 to 0.80). This finding is supported by findings of previous studies, where the efficacy of CCR was limited for treatment of non-SCCA of the cervix, suggesting that non-SCCA was a poor prognostic factor when compared with SCCA [20]. Thus, CCR can be considered as more effective in patients with stage IVB SCCA of the cervix.
However, the current study has some limitations, as follows: a retrospective review of medical records; a small number of enrolled patients; various doses and extents of RT; different chemotherapeutic regimens; the selection of treatment methods based on physician decision between SC and CCR, which may reduce statistical power to interpret these findings.
ConclusionCCR may be more effective for improving survival than SC, which can be considered as a feasible method with some caution regarding late RT-related toxicity for treatment of stage IVB cervical cancer with distant lymphatic metastasis. Thus, findings of the current study suggest that conduct of prospective clinical trials for the efficacy of CCR for treatment of stage IVB cervical cancer with distant lymphatic metastasis may be worthwhile.
AcknowledgmentsThis research was supported by a grant (No. 04-2012-0890; 03-2012-0170) from the Seoul National University Hospital (SNUH) research fund, and the Korean Gynecologic Cancer Foundation.
References1. Suh DH, Kim K, Kim JW. Major clinical research advances in gynecologic cancer in 2011. J Gynecol Oncol. 2012;23:53–64. PMID: 22355468
2. National Comprehensive Cancer NetworkCervical cancer clinical practice guidelines in oncology (v.I.2010) [Internet]. Fort Washington: National Comprehensive Cancer Network; cited 2012 Nov 1Available from: http://www.nccn.org
3. Pectasides D, Kamposioras K, Papaxoinis G, Pectasides E. Chemotherapy for recurrent cervical cancer. Cancer Treat Rev. 2008;34:603–613. PMID: 18657909
4. Yoo HJ, Lim MC, Seo SS, Kang S, Yoo CW, Kim JY, et al. Pelvic exenteration for recurrent cervical cancer: ten-year experience at National Cancer Center in Korea. J Gynecol Oncol. 2012;23:242–250. PMID: 23094127
5. Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group Study. J Clin Oncol. 2004;22:3113–3119. PMID: 15284262
6. Monk BJ, Sill MW, McMeekin DS, Cohn DE, Ramondetta LM, Boardman CH, et al. Phase III trial of four cisplatin-containing doublet combinations in stage IVB, recurrent, or persistent cervical carcinoma: a Gynecologic Oncology Group Study. J Clin Oncol. 2009;27:4649–4655. PMID: 19720909
7. Saito I, Kitagawa R, Fukuda H, Shibata T, Katsumata N, Konishi I, et al. A phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB, persistent or recurrent cervical cancer: Gynecologic Cancer Study Group/Japan Clinical Oncology Group Study (JCOG0505). Jpn J Clin Oncol. 2010;40:90–93. PMID: 19825815
8. Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001;478:23–43. PMID: 11406167
9. Hill BT. Differing patterns of cross-resistance resulting from exposures to specific antitumour drugs or to radiation in vitro. Cytotechnology. 1993;12:265–288. PMID: 7765329
10. Nishio S, Katsumata N, Matsumoto K, Tanabe H, Yonemori K, Kohno T, et al. Analysis of the clinicopathological prognosis of stage IVb cervical carcinoma. Oncol Rep. 2008;19:497–503. PMID: 18202800
11. Kim JY, Kim JY, Kim JH, Yoon MS, Kim J, Kim YS. Curative chemoradiotherapy in patients with stage IVB cervical cancer presenting with paraortic and left supraclavicular lymph node metastases. Int J Radiat Oncol Biol Phys. 2012;84:741–747. PMID: 22898382
12. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. PMID: 10655437
13. Common Terminology Criteria for Adverse Events (CTCAE) v3.0 [Internet]. Bethesda: National Cancer Institute; cited 2012 Nov 1Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_30
14. Sakurai H, Mitsuhashi N, Takahashi M, Akimoto T, Muramatsu H, Ishikawa H, et al. Analysis of recurrence of squamous cell carcinoma of the uterine cervix after definitive radiation therapy alone: patterns of recurrence, latent periods, and prognosis. Int J Radiat Oncol Biol Phys. 2001;50:1136–1144. PMID: 11483322
15. Xiao ZF, Yang ZY, Miao YJ, Wang LH, Yin WB, Gu XZ, et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys. 2005;62:82–90. PMID: 15850906
16. Lee SH, Lee SH, Lee KC, Lee KB, Shin JW, Park CY, et al. Radiation therapy with chemotherapy for patients with cervical cancer and supraclavicular lymph node involvement. J Gynecol Oncol. 2012;23:159–167. PMID: 22808358
17. Kim HS, Kim JY, Park NH, Kim K, Chung HH, Kim YB, et al. Matched-case comparison for the efficacy of neoadjuvant chemotherapy before surgery in FIGO stage IB1-IIA cervical cancer. Gynecol Oncol. 2010;119:217–224. PMID: 20705335
18. Jhingran A. Potential advantages of intensity-modulated radiation therapy in gynecologic malignancies. Semin Radiat Oncol. 2006;16:144–151. PMID: 16814154
19. Rose PG, Blessing JA, Gershenson DM, McGehee R. Paclitaxel and cisplatin as first-line therapy in recurrent or advanced squamous cell carcinoma of the cervix: a gynecologic oncology group study. J Clin Oncol. 1999;17:2676–2680. PMID: 10561341
20. Narayan K, Fisher RJ, Bernshaw D, Shakher R, Hicks RJ. Patterns of failure and prognostic factor analyses in locally advanced cervical cancer patients staged by positron emission tomography and treated with curative intent. Int J Gynecol Cancer. 2009;19:912–918. PMID: 19574784
Fig. 1Kaplan-Meier analyses with the log-rank or Breslow test for progression-free and overall survival in 24 patients with stage IVB cervical cancer: (A) chemoradiation (CCR) vs. systemic chemotherapy (SC), (B) squamous vs. non-squamous cell carcinoma, (C) complete response (CR) vs. non-CR. 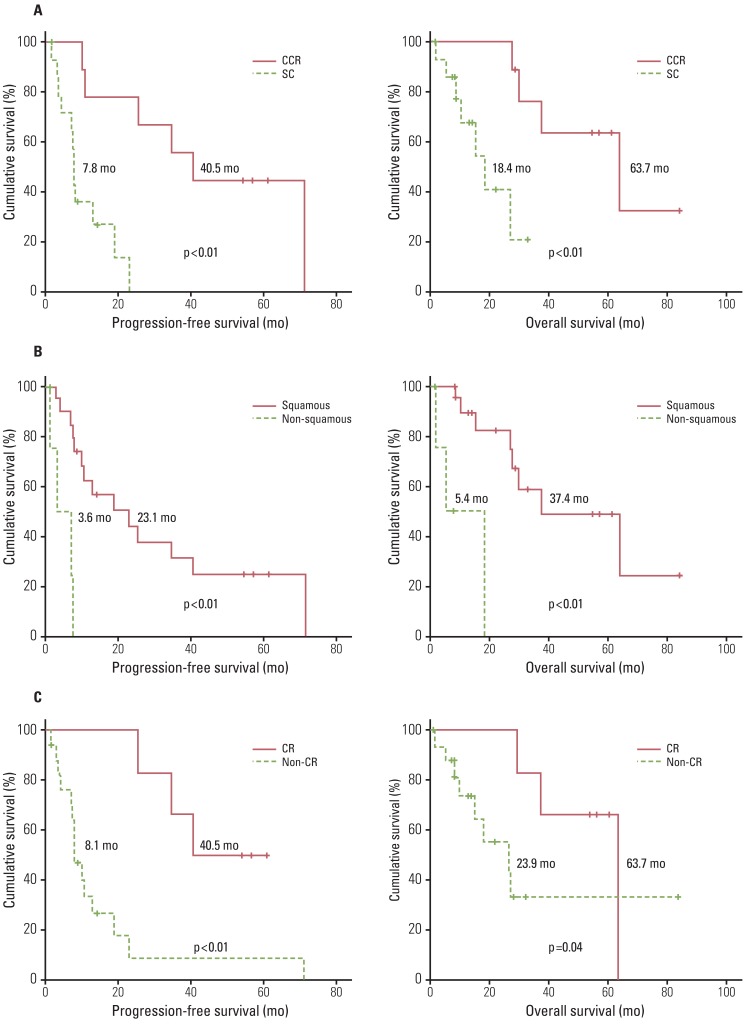
Table 1Clinicopathologic characteristics Table 2Results of 10 patients with FIGO stage IVB cervical cancer who received concurrent chemoradiation Table 3Grade 3 or 4 acute hematological and late radiotherapy (RT)-related toxicities Table 4Favorable prognostic factors for survival by multivariate Cox's proportional hazard analysis in patients with FIGO stage IVB cervical cancer Table 5.Disease recurrence in FIGO stage IVB cervical cancer Table 5Disease recurrence in FIGO stage IVB cervical cancer |
|
||||||||||||||||||||||||||||||||||||||||||||