INTRODUCTION
Numerous tumor markers that are produced and secreted by tumors are currently being utilized in the diagnosis, treatment, and follow up aspects of cancer. Tumor markers that are employed in uterine cervical cancer are CEA (Carcino-embryonic antigen) and CA-125 (Cancer antigen-125) for adenocarcinomas, and SCC (Squamous cell carcinoma) antigen and Cyfra 21-1 for squamous cell carcinoma (1).
The SCC antigen is known to assist in the staging of cervical cancer, as well as providing information with regard to tumor dissemination and recurrence (2). However, the lower than desired sensitivity and specificity values of SCC antigen limits the degree of clinical applicability, and therefore continuous effort has been put in over the years to find new tumor markers that will provide accurate and essential clinical information to the physician (3).
Cytokeratin is an intermediate filament that is a part of the cytoskeleton secreted by both normal epithelium and cancer tissues. From cervical cancer and normal cervix cells, cytokeratin specific antibodies have been elucidated by immunohistochemistry techniques. Thereafter, Cyfra 21-1, an acidic subunit of cytokeratin 19, was separated from the human serum by a new immunoradiometric assay method (4). Cyfra 21-1 has been utilized as a tumor marker mainly in non-small cell carcinoma of the lungs and in other types of squamous cell tumors. In terms of efficacy in the diagnosis of cervical cancers, Cyfra 21-1 has been shown to have a lower sensitivity compared to SCC antigen, but it has been reported to provide information regarding tumor size and lymph node status. Also, measurement of Cyfra 21-1 in conjunction with SCC antigen levels have been shown to enhance diagnosis accuracy and recurrence prediction (5~7). Past literature has demonstrated that in patients with cervical cancer, pre-treatment levels of SCC antigen and Cyfra 21-1 is associated with FIGO staging, tumor size, and lymph node involvement. In addition, the combination of these two tumor markers have been reported to be related to tumor recurrence and survival after surgical or irradiation therapy (8~11).
The aim of this study was to determine the relationship between pretreatment serum SCC antigen/Cyfra 21-1 levels and survival in patients with invasive squamous cell carcinoma of the cervix.
MATERIALS AND METHODS
1) Materials
From September 1994 to December 2000, 131 patients with squamous cell carcinoma of the uterine cervix who were diagnosed and treated at our institution were enrolled in this study. These patients underwent measurement of pre-treatment SCC antigen and Cyfra 21-1, and were followed up for a period of at least 5 years. All patients were histologically confirmed as squamous cell cancer and all cases of adenocarcinoma or adenosquamous cell types were excluded. At 5 years of follow-up, there were 32 patients who had recurrent disease (RD), and 99 patients who had no recurrent disease (NRD).
2) Methods
Pre-treatment serum SCC antigen was measured by IMx System SCC (Abbott Diagnostics, Chicago, Ill, USA) which automatically determines SCC antigen levels by Microparticle Enzyme Immunoassay (MEIA). Serum Cyfra 21-1 levels were measured by Solid-phase sandwich-type immunoradiometric assay (IRMA, CIS Biointernational, France; CIS Diagnostic KK, Japan).
Follow-up after primary therapy (surgery only, surgery+radiotherapy, surgery+concurrent chemoradiotherapy, concurrent chemoradiotherapy only, radiotherapy only) was up to January of 2005 for a mean of 55 months (6~104 months). Follow-up consisted of bimanual pelvic and physical examination, serum SCC antigen and Cyfra 21-1 measurements, and Pap smears every 3 months during the first year of follow-up, then every 6 months in the year after, and then once a year thereafter. Suspicious recurrent disease was confirmed by biopsy, and determination of tumor size changes by computerized tomography (CT scans) or magnetic resonance imaging (MRI) techniques.
Complete response after therapy was defined as absence of clinical disease by the above imaging techniques for 4 weeks after completion of primary therapy, and partial response was defined as more than 50% reduction in tumor size after 4 weeks without evidence of any new lesions. Stable disease was defined as less than 50% reduction or less than 25% increase in tumor size, while progressive disease was when there was evidence of new lesions after 4 weeks. Recurrent disease was defined as a newly developed lesion that occurred 6 months after therapy and complete response. Persistent disease was defined as occurrence of disease within 6 months of primary therapy, and these patients were excluded from this study.
The 2 study groups, RD and NRD groups were then compared with respect to pre-treatment serum SCC antigen and Cyfra 21-1 levels, FIGO stage, tumor size, lymph node metastasis, and parametrial involvement. We also determined the time interval from completion of therapy to recurrence of disease, site of recurrence, and the relationship between serum SCC antigen/Cyfra 21-1 levels and survival and recurrence.
3) Statistical analysis
Statistical analysis of observed data was performed by utilizing SPSS for Windows (Version 11.5, SPSS Inc, Chicago, Ill, USA). T-test, chi-square test, and correlation coefficients were applied for relationship between serum SCC antigen/Cyfra 21-1 levels and clinical characteristics in the RD and NRD groups. ROC curves were analyzed for determining sensitivity, specificity, positive and negative predictive values for serum SCC antigen/Cyfra 21-1 levels in predicting disease. The Kaplan-Meier method and Cox regression analysis was employed for survival rates. p values less than 0.05 were considered to be statistically significant.
RESULTS
The characteristics of the 131 patients enrolled in this study are shown in Table 1. We were able to observe that the pre-treatment levels of SCC antigen in the NRD and RD groups were 7.45±18.54 ng/ml and 28.98±49.15 ng/ml respectively, while for Cyfra 21-1 it was 2.84±4.41 ng/ml and 11.72±15.11 ng/ml respectively, showing that both pre-treatment SCC antigen and Cyfra 21-1 levels were significantly higher in the RD group (p<0.001, Table 1).
When the above tumor markers were statistically analyzed with respect to each stage of disease, we were not able to discern any difference between the four stages of cervical cancer (data not shown). However when the stages were divided into two groups, an early stage group consisting of surgically treated stages Ib-IIa and a late or advanced group consisting of non-surgically treated stages IIb-IVb, we found that there was significant difference in the levels of the respective tumor markers between the NRD and RD patients (Table 1).
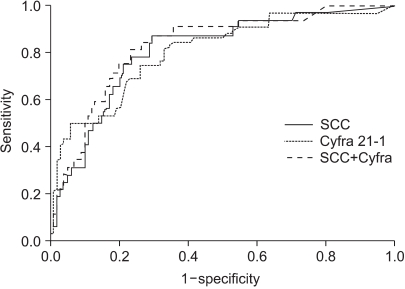
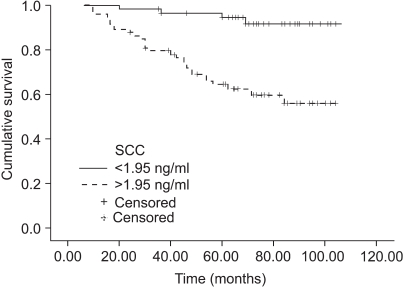
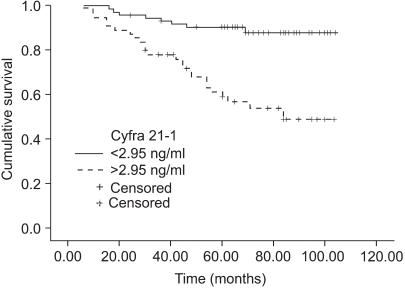
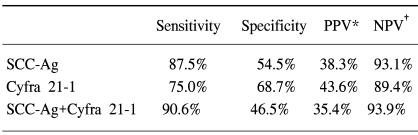
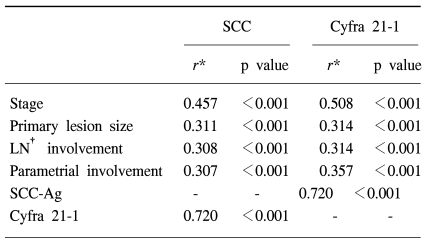
The normal levels of serum SCC antigen and Cyfra 21-1 were determined as 1.95 ng/ml and 2.95 ng/ml, respectively, from the ROC curves (Fig. 1). The sensitivity, specificity, positive predictive, and negative values for SCC antigen alone in predicting recurrent disease, was 87.5%, 54.5%, 38.3%, 93.1%, while for Cyfra 21-1 alone it was 75.0%, 68.7%, 43.6%, 89.4%. When SCC antigen and Cyfra 21-1 were both employed, the values were 90.6%, 46.5%, 35.4%, 93.9%, showing increased sensitivity compared to SCC or Cyfra 21-1 alone (Table 2). There was significant relationship between pre-treatment serum SCC antigen/Cyfra 21-1 levels and high FIGO stage, large tumor size, positive lymph node metastasis, and parametrial involvement (Table 3).
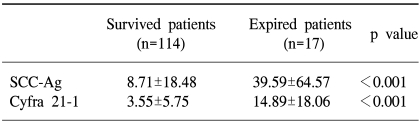
The sites of recurrent disease was categorized according to the classification described by Krebs in 1982, and in our study there were 9 cases of central recurrence, 5 cases in the pelvic side wall, and 18 cases that were distant recurrences. Among the latter, lymph nodes were the most common (10), followed by the lungs (4), peritoneal surfaces (3), and bone (1). There was statistically significant difference in the pre-treatment SCC antigen and Cyfra 21-1 levels according to the different sites of recurrence (p=0.04, 0.01), but no difference was found in the aforementioned levels at the time of recurrence (Fig. 2, 3). When the same markers were compared between survived and expired patients (at the time of writing), it was shown that both markers were significantly increased in the expired patients group. On the other hand, no difference in tumor markers was observed levels between the survived and expired patients at the time of recurrence (not shown) (Table 4).
The mean survival for the RD group was 49.86 months, the 2 year survival rate was 68.75%, and the 5 year survival rate was 15.62%. There was no relationship between pre-treatment SCC antigen/Cyfra 21-1 levels and levels at the time of recurrence, and the time interval until recurrence. The calculated odds ratio for pre-treatment SCC antigen and Cyfra 21-1 on recurrent disease and survival were 6.87 and 5.07, respectively, which were both statistically significant (Table 5).
DISCUSSION
In the Korean population, cancer of the uterine cervix is second only to breast cancer in terms of incidence, and is characterized by relatively high survival rates when diagnosed and treated in the early stages. But despite continued efforts in early diagnosis and treatment, the overall survival rate has not increased markedly, and which is attributed to recurrent or persistent disease. Therefore, in order to enhance survival, it is essential to implement regular follow-up and hence detect early recurrent or persistent disease, to which end various tumor markers have been utilized to date (1~14).
One of the major tumor markers is the SCC antigen, a subfraction of the glucoprotein Tumor Antigen-4 (TA-4) that was first elucidated from squamous cancer cells by Kato and Torigoe in 1977 (2), and which is currently widely employed as a tumor marker in both keratinizing and non-keratinizing cell types of squamous cell carcinoma of the uterine cervix. It has been reported in the previous literature that pre-treatment levels of SCC antigen is associated with FIGO stage, lesion size, and parametrial involvement (1,3,11), and that continued or increasing levels of SCC antigen reflects persistent or recurrent disease, and predicts survival (1,3).
However, several studies have also shown that initial SCC antigen levels are elevated in only 24~53% of FIGO stage Ib or IIa squamous cell cancer patients, suggesting that SCC antigen is not appropriate in the early diagnosis of cervical cancers (3,15). In terms of lymph node metastasis, Duk et al demonstrated that elevated pre-treatment SCC antigen levels is an independent factor in squamous cell cancer patients (12), while Gaarenstroom et al proposed that there was no relationship between SCC antigen levels at the time of diagnosis and lymph node metastasis or survival (9). It has also been reported that normal levels of SCC antigen during follow-up cannot exclude persistent or recurrent disease (1,16). The information above therefore suggests that the SCC antigen plays a limited clinical role in screening and post-treatment follow-up of cervical cancer. Efforts to find other tumor markers besides SCC antigen have led to the discovery of cytokeratin 19 by immunohistochemistry techniques, which is also expressed in squamous cell cancers of the uterine cervix (17). By Solid-phase enzyme linked sandwich immunoradiometric assay (IRMA) techniques, the subfraction Cyfra 21-1 has been shown to demonstrate a high diagnostic sensitivity and specificity for squamous cell cancers, especially in non-small cell cancer of the lungs (4).
In cervical cancers, several authors have supported the role of Cyfra 21-1 as a tumor marker. Bonfrer et al reported that pre-treatment SCC antigen levels were closely related to FIGO stage and lesion size in a study of 78 squamous cell cancer patients, and that elevated Cyfra 21-1 levels correlated with poor prognosis, suggesting that Cyfra 21-1 be employed as a supplemental tumor marker in combination with serum SCC antigen (18). Other authors have also suggested the significance of pretreatment Cyfra 21-1 levels as a predictor of extent of disease, prognostic factor, and its role in ascertaining recurrent disease (5~7). Although Cyfra 21-1 levels increase with higher FIGO stage, the sensitivity and specificity for diagnosis of squamous cell cancers is limited in that it remains lower than that of serum SCC antigen (15), leading to attempts to combine serum SCC antigen and Cyfra 21-1 to enhance sensitivity and specificity. In a previous study by the authors, when the cut-off values of serum SCC antigen and Cyfra 21-1 was set at 1.94 ng/ml and 3.1 ng/ml, the sensitivity for SCC antigen was 55%, 45% for Cyfra 21-1, and 62% when both were combined, showing increased sensitivity in the diagnosis of cervical cancers (11).
A previous report by Gaarenstroom, et al showed that patients with elevated values of serum SCC antigen have a higher risk of persistent or recurrent disease compared to elevated levels of Cyfra 21-1 alone (19), while Kainz et al reported the sensitivity, specificity, positive and negative predictive values in the detection of recurrence of cervical cancer as 66%, 91%, 93%, 60% for SCC antigen alone, 63%, 96%, 96%, 59% for Cyfra 21-1 alone, while for SCC antigen+Cyfra 21-1 it was 78%, 87%, 91%, 63%, suggesting that detection of cervical cancer recurrences with SCC is improved by the combination with Cyfra 21-1 (5). Other authors have similarly reported results that are in agreement with the above result (6,7). In our study, we were able to observe similar results in that the pre-treatment value sensitivity for recurrent disease for SCC alone was increased to 90.6% when both tumor markers were employed.
Although Gaarenstroom et al suggested a relationship between pre-treatment Cyfra 21-1, TPA, SCC antigen levels and tumor size, and concluded that this was insufficient for identifying patients at risk for the presence of lymph node metastases or parametrial involvement (9), Taketa et al stated that pre-treatment analysis of the DTM index (double-tumor marker index) of SCC and CA125 levels allows estimation of lymph node status and prognosis for patients with cervical squamous cell carcinoma (13). In this study we were able to further these findings by showing that there was a significant relationship between the two tumor markers and the prognostic factors FIGO stage, lesion size, lymph node metastasis, and parametrial involvement.
The relationship between a number of tumor markers and survival is as yet unclear and equivocal, as evidenced by previous studies. Pre-treatment SCC antigen, Cyfra 21-1, and tissue polypeptide antigen (TPA) levels have been suggested to be unrelated with survival (20), while other studies have suggested that FIGO stage is a more significant factor for survival compared to pre-treatment SCC antigen, TPA, or Cyfra 21-1 levels (21). In another study by Duk et al in a large series of 653 squamous cell cancer patients, they demonstrated that especially in early stage disease, pre-treatment SCC antigen level was an independent prognostic factor of survival. Also, even in the absence of lymph node metastasis high levels of pre-treatment leads to a 3-fold increase in risk for recurrent disease (12).
CONCLUSIONS
The authors suggest that initial pre-treatment levels of serum SCC antigen and Cyfra 21-1 are closely related to FIGO stage, lesion size, lymph node and parametrial involvement in patients with squamous cell carcinoma of the cervix. Also, these markers are of value in early prediction of recurrent disease and survival rates.