AbstractPurposeAs the survival of head and neck cancer (HNC) improves, survivors increasingly confront non-cancer–related deaths. This nationwide population-based study aimed to investigate non-cancer–related deaths in HNC survivors.
Materials and MethodsData from the Korean Central Cancer Registry were obtained to characterize causes of death, mortality patterns, and survival in patients with HNC between 2006 and 2016 (n=40,890). Non-cancer-related mortality relative to the general population was evaluated using standardized mortality ratios (SMRs). The 5- and 10-year cause-specific competing risks probabilities of death (cumulative incidence function, CIF) and subdistribution hazards ratios (sHR) from the Fine-Gray models were estimated.
ResultsComorbidity-related mortality was frequent in older patients, whereas suicide was predominant in younger patients. The risk of suicide was greater in patients with HNC than in the general population (SMR, 3.1; 95% confidence interval [CI], 2.7 to 3.5). The probability of HNC deaths reached a plateau at 5 years (5-year CIF, 33.9%; 10-year CIF, 39.5%), whereas the probability of non-HNC deaths showed a long-term linear increase (5-year, CIF 5.6%; 10-year CIF, 11.9%). Patients who were male (sHR, 1.56; 95% CI, 1.41 to 1.72), diagnosed with early-stage HNC (localized vs. distant: sHR, 1.86; 95% CI, 1.58 to 2.21) and older age (65–74 vs. 0–44: sHR, 6.20; 95% CI, 4.92 to 7.82; ≥ 75 vs. 0–44: sHR, 9.81; 95% CI, 7.76 to 12.39) had an increased risk of non-cancer mortality.
IntroductionCompeting mortality is becoming more important in cancer survivors. Comorbidities, treatment-related morbidities, frailty, and old age are often related to ‘non-cancer’ competing mortalities in cancer patients, including patients with head and neck cancer (HNC) [1,2]. As the survival of HNC has recently been improving [3–5], an increasing number of survivors constitutes the aged population [6]. An in-depth understanding of competing non–cancer causes of death (COD) and corresponding mortality risks in survivors of HNC became necessary.
Patients with HNC may experience a higher risk of competing (non-HNC) mortalities than the general population [2,7–11], and the survivors frequently experience comorbidities such as pneumonia and lower respiratory disease and are at an increased risk of competing mortalities [1,7,12–17]. Advanced stage diseases require intensive combined treatments, which could cause related morbidities, usually in the frail elderly, in various cancers, such as human papillomavirus-positive, -negative oropharyngeal carcinoma, and oral carcinoma [17–21]. Furthermore, HNC is often accompanied by psychosocial morbidities, leading to intentional self-harm (suicide) [22]. Meanwhile, a recent report has debated that HNC did not have the greatest risk of suicide, especially after the emergence of human papillomavirus-associated HNC [23]. These features have yet to be clarified in Korea.
Despite the importance of a comprehensive study on competing mortality, previous nationwide population-based studies, focused mainly on the incidence trends, have not sufficiently highlighted non-cancer competing mortalities and their long-term survival statistics in patients with HNC, especially in the Asian population, for example, in South Korea [24,25]. Moreover, several western population–based studies [2,6,8] and single institute-based clinic studies exist [26,27]; however, these were only focused on the risk factor assessment, but not age-specific long-term competing mortality patterns. Therefore, we aimed to investigate the survivorship and non-cancer–related COD in HNC survivors in Korea and provide age-specific non-cancer mortality patterns and long-term survival statistics. We also included the impact of the human papillomavirus (HPV) association on non-cancer mortality. Detailed knowledge about the pattern of non-cancer COD in HNC patients could enhance current survivorship and surveillance strategy, important for reasonable survivorship guidelines.
Materials and Methods1. Study populationWe obtained data from the Korea Central Cancer Registry (KCCR), a population-based cancer registry. The KCCR is a nationally representative, population-based cancer registry including more than 99% of cancer patients in South Korea and contains the nationwide cancer incidence and survival data dating back to 1999. The KCCR registry is linked to the mortality and COD statistics provided by Statistics Korea, a National Statistics Office in South Korea [28]. The COD statistics were obtained from death certificates and classified according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10), recommended by the World Health Organization (WHO) [29,30].
Patients diagnosed with malignant primary head and neck tumors between January 1, 2006, and December 31, 2016, were included in this study and followed until December 31, 2017. We included the first primary HNC (HNC only or first of multiple primaries, both male and females, all ages). We defined HNC based on the International Classification of Diseases for Oncology (ICD-O, 3rd edition) codes, including C000–C009 (lip), C019–C029 (tongue), C079–C089 (salivary gland), C040–C049 (floor of mouth), C030–C039, C050–C059, C060–C069 (gum and other mouth), C110–C119 (nasopharynx), C090–C099 (tonsil), C100–C109 (oropharynx), C129, C130–C139 (hypopharynx), C140, C142, C148 (other oral cavity and pharynx), C300–C301, C310–C319 (nose, nasal cavity, and middle ear), and C320–C329 (larynx). HNC was further classified as HPV-associated sites (C090–C099, C019, C051–C052, and C100–C109), HPV-unrelated sites (C020–C069, C129–C139, and C320–C329), and other sites (C000–C009, C058–C059, C079–C089, C110–C119, C300–C301, C310–C319, and C140–C148). The exclusion criteria for this study were as follows: (1) cases with missing COD information (< 1%) or with death certificate only; (2) cases with multiple primary tumors; and (3) cases lost to follow-up. The study protocol was approved by the Institutional Review Board of the National Cancer Center (NCC2016-0041) of Korea.
2. Cause of death classificationWe used underlying COD recorded as ICD-10 codes and applied the cause-specific death classification algorithm to reclassify COD in patients with cancer. This algorithm was developed and validated in the United States National Cancer Center Institute’s Surveillance Epidemiology and End Results (SEER) program to improve the accuracy of COD information and correct possible misclassifications [31,32]. In our study, we classified the COD as either HNC or other causes, where the latter category included cancers other than HNC and non-cancer causes. To determine COD rankings in cancer patients and estimate non-cancer mortality risks relative to the general population, we classified 56 COD selected from a set of 80 COD recommended by the WHO and used by Statistics Korea [30].
3. Statistical analysisDescriptive statistics were used to analyze the demographics, tumor characteristics, and proportion of deaths according to COD among patients with HNC. The distribution of COD was analyzed by sex, age group (0–44, 45–54, 55–64, 65–74, ≥ 75), and tumor stage (localized, regional, and distant) at diagnosis. We estimated standardized mortality ratios (SMRs) and 95% confidence intervals (CIs) to compare non-cancer mortality risks in patients with HNC relative to the general South Korean population. The 95% CI for the SMR was calculated using the method described by Kahn and Sempos [33]. Observed all-cause survival probabilities were estimated using Kaplan-Meier methods. To estimate the probability of death by HNC compared to death by other competing causes under the competing risks, we used the cumulative incidence function (CIF) [34]. Survival probabilities and CIFs were estimated over the follow-up years (up to 12 years) stratified by age groups (0–44, 45–54, 55–64, 65–74, and ≥ 75) and tumor stages (localized, regional, and distant). Gray’s test was used for the CIF comparisons [35]. Fine-Gray competing risks survival models were fitted [36]. The subdistribution hazard ratios were used to evaluate the association between patient characteristics, including sex, age group, tumor stage, and HPV association with the risk of death from HNC or other causes. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC). p-values less than < 0.05 were considered statistically significant.
Results1. Causes of deathOf the 40,890 patients included in the study, 16,730 (41%) died (males: 13,596; females: 3,134). Of these, 13,767 patients (82%) died from HNC, and 2,963 patients (18%) died from other causes (Table 1). Of all non-HNC–related COD, cancer of other sites comprised 27% (n=807), whereas the other 73% were non-cancer–related causes. The most frequent non-cancer–related COD was heart disease (n=265, 9%), followed by suicide (n=256, 9%), cerebrovascular diseases (n=227, 8%), pneumonia (n=185, 6%), and chronic lower respiratory diseases (n=109, 4%). Death by medical comorbidities, such as pneumonia and heart and cerebrovascular disease, were more predominant in older patients. In contrast, suicide was the most frequent non-cancer–related COD in younger patients (< 65 years).
2. Standardized mortality ratioThe SMRs of the most frequent non-HNC–related COD are shown in Table 2. The SMRs of suicide (SMR, 3.1; 95% CI, 2.7 to 3.5), pneumonia (SMR, 1.7; 95% CI, 1.4 to 1.9), and chronic lower respiratory diseases (SMR, 1.3; 95% CI, 1.1 to 1.6) were significantly higher in patients with HNC than the general population of South Korea. Notably, the risk of suicide was higher in both males and females (SMR in males, 2.3 [95% CI, 2.0 to 2.6]; SMR in females, 2.5 [95% CI, 1.5 to 3.4]). Additionally, in male patients with HNC, suicide was the most frequent non-cancer-related COD (n=229).
3. Survival and competing mortality risks: HNC versus non-HNC–relatedThe proportions (probabilities) of surviving patients and those who died from HNC and competing non-HNC (other) causes are shown in Fig. 1. Although the proportion of surviving patients declined for all COD, non-HNC mortality gradually increased for prolonged follow-up periods and increased more rapidly in older populations. In the presence of competing risks, the probability of death by HNC tended to reach a plateau at approximately 5 years, whereas death from causes other than HNC maintained a steady linear increase up until 10 years, with significantly higher mortality in the elderly (p < 0.001). In the elderly with early-stage HNC, the risk of death from non-HNC-related causes became comparable to the probability of dying from HNC (Figs. 1 and 2). The chances of competing non-HNC (other) deaths were higher in older than in younger patients. Non-HNC death was more predominant in localized SEER stage patients than in the advanced, most of whom died from HNC (Fig. 2). Meanwhile, the HNC was constantly the most common COD in all age groups. Non-HNC mortality linearly increased throughout the follow-up, and this pattern did not change by HPV status (S1 Fig.).
The 5- and 10-year survival and probability of death by HNC and other competing causes are shown in Table 3. In both males and females, the 5- and 10-year survival probabilities were 60.5% and 48.6%, respectively. The probability of death by HNC was 33.9% in 5 years and 39.5% in 10 years of follow-up, whereas the probability of death by competing non-HNC causes was 5.6% in 5 years and increased to 11.9% in 10 years of follow-up. For patients with early-stage cancer, the probability of non-HNC mortality increases as they survive. For example, in patients with a localized tumor, the 5- and 10-year probabilities of non-HNC mortality were 6.1% and 14.0%, respectively. However, for a patient with advanced SEER stage cancer, regardless of age group, the 5- and 10-year probabilities of death by HNC were higher (5-year, 72.4%; 10-year, 77.8%), whereas probabilities of death by non-HNC causes were lower (5-year, 4.1%; 10-year, 6.2%).
The Fine-Gray competing risks modeling results in Table 4 showed that the risks of death were elevated in the male and older ages for both HNC (male vs. female: subdistribution hazards ratio [sHR], 1.19 [95% CI, 1.14 to 1.25]; age 65–74 vs. 0–44: sHR, 2.64 [95% CI, 2.45 to 2.84]) and non-HNC deaths (male vs. female: sHR, 1.56 [95% CI, 1.41 to 1.72]; age 65–74 vs. 0–44: sHR, 6.20 [95% CI, 4.91 to 7.82]). Age had a substantial impact on non-HNC mortality. Patients diagnosed with early-stage tumors had an increased risk of non-cancer mortality (localized vs. distant: sHR, 1.86 [95% CI, 1.58 to 2.21]). Whereas, for patients diagnosed with a distant stage, the hazard of HNC death was more substantial (distant vs. localized: sHR, 6.71 [95% CI, 6.34 to 7.11]). HPV association was related to the risks of HNC deaths (p < 0.001) but not to the risks of non-HNC deaths (p=0.683).
The probability and risks of suicide were provided in S2 and S3 Tables. In both males and females, the 5- and 10-year probability of suicide was 0.6% and 0.8%, respectively. In the males, the probability of suicide was elevated up to 1% in 10 years (S2 Table). The risks of suicide were 2 times higher in males (male vs. female: sHR, 2.35 [95% CI, 1.57 to 3.50]) and patients diagnosed with regional stage (regional vs. distant: sHR, 1.78 [95% CI, 1.00 to 3.15]) (S3 Table).
DiscussionWe herein described survival statistics and competing mortality patterns, particularly non-cancer–related deaths in HNC, using nationwide population-based data in South Korea. In patients with HNC, the risk of cancer deaths was still high, but non-cancer deaths were continuously increasing. Non-cancer deaths associated with comorbidities were frequent in the elderly (≥ 65), whereas intentional self-harm was predominant in the younger (< 65) male population. Our results demand raising awareness regarding proper survivorship for HNC survivors. Targeting both comorbidities and psychosocial aspects for HNC survivors seems important.
Although the previous study reported that death by competing causes was more frequent than HNC itself [11,37,38], our study showed that the probability of dying from other causes was lower than HNC, even after ≥ 10 years of follow-up, regardless of sex, age, and tumor stage. However, the risk of non-cancer COD increased consistently long after the treatment. In contrast, the probability of HNC death reached a plateau at 5 years. Non-cancer deaths increased in older age and early stage. Comorbidities, age, and intentional self-harm were predominant non-caner causes. Our results were consistent with the SEER data [2,8], which reported cardiovascular disease and pneumonia as the leading non-cancer deaths. Studies from different countries have shown various patterns of competing mortality [6,8,10,11,26,39,40].
Traditionally, age and comorbidities have been determinants of both HNC- and non-cancer mortality [26,27,39,41,42]. Accordingly, our findings showed that non-cancer deaths sharply increased by age (2.8%, 4.3%, 7.3%, and 12% for age groups 45–54, 55–64, 65–74, and 75 or older, respectively). Additionally, non-cancer deaths by pneumonia, chronic lower respiratory diseases, and heart disease were predominant in the elderly. Cardiovascular disease and stroke were also major causes. Previous studies also showed elevated risks of fatal stroke and heart diseases among HNC patients [6,43,44]. Considering that Asian countries such as South Korea have rapidly aging populations [45,46], our data demand awareness and plans, especially for the frail elderly.
Intentional self-harm was a primary cause of non-cancer death in HNC survivors. Risks of suicide in patients with HNC have generally been reported to be significantly higher than in other sites [47], although this tendency is recently being debated after the emergence of HPV-associated HNC [23]. Meanwhile, our data indicated that HNC survivors had higher mortality from intentional self-harm, 3 times higher than the general population. The risk of suicide among patients with HNC was known to be double that of patients with cancer of other sites and about 4 times that of the general population [47]. Compared to the previously reported risk of suicide in some cancers in South Korea [48], the risk of intentional self-harm in HNC (SMR, 3.1; male, 2.3; female, 2.5) was higher than stomach or colorectal but lower than lung cancer. In particular, risks were higher in the younger (age < 65) males. Higher mortality risk than the general population in South Korea, where the background suicide rates are already high [49,50], seems concerning. HNC survivors endure unique clinical conditions. HNC has been known to be the most emotionally and psychosocially stressing of all cancers [51]. Sources of distress are persistent effects of treatment, difficulty swallowing and airway care, depression, functional compromise, and esthetic disfigurement [52,53]. Pain and substance abuse are also known to be more prevalent [54]. Considering emotional, esthetic, functional, and eventual psychosocial distress caused by HNC [52,53,55], better psychosocial survivorship strategies and policies are warranted to prevent self-harm.
The pattern of mortality including intentional self-harm in HPV-associated cancers was similar to HPV-unrelated cancers, contrasted to the previous speculation that recent decreasing suicide rate may be secondary to the increasing distribution of HPV-associated HNC, and a decrease in those with tobacco and alcohol [23]. We showed that the risk of HNC and competing deaths tended to be lower in HPV-related than HPV-unrelated cancers, albeit not substantial. Considering occurrence in the healthier and younger population and better survival of HPV-related cancer [55], the risk of death by HPV-associated cancer might continue to remain low, whereas competing death risk is expected to elevate. Yet, differences by HPV association did not appear significantly in this study. Considering the adoption of a de-intensified treatment for HPV-associated cancer may mitigate this pattern in the future, as well as related, debating data [23], follow-up analysis seems in need.
HNC survivors undergo potentially significant effects from cancer and its treatment and deserve comprehensive and coordinated clinical follow-up care. Patients should be provided adequate support to address physical, psychosocial, and practical effects after treatment. In regard, HNC survivorship care guidelines by the American Cancer Society stated that HNC survivors also need to be counseled on health promotion strategies to minimize and mitigate long-term effects and comorbid health conditions to potentially increase survival [56]. In particular, comprehensive assessment and counseling about psychosocial effects, including body and self-image issues, and distress/depression/anxiety, was recommended with a moderate level of evidence. Considering that these aspects have not been addressed enough in Korea, adequate related guidelines or policymaking seems necessary.
This study has several limitations. Although comprehensive, our population-based cancer registry data lacks detailed prognostic factors and clinical information, including p16, HPV test results, treatment information, and TNM stage. Predictive modeling was difficult due to the lack of information. Developing predictive prognosis models that incorporate detailed clinical information in electronic health records regarding significant predictors for survival in patients with HNC will be followed. While we showed that death from SPM, with the most common being lung, stomach, and liver cancer, similar to the SEER data [2], in-depth mortality analysis focusing on SPM was beyond the scope of this study. Finally, misclassification of the cause of death based on death certificates might exist and bias the survival estimates. However, we used cause of death information from Statistics Korea, where the official cause of death statistics is reported annually in South Korea [30]. Statistics Korea has made ongoing efforts to improve accuracy in the cause of death data collection. We further applied the SEER cause-specific death classification algorithm to enhance the accuracy of cause of death classifications and survival estimates in cancer patients [32]. Nevertheless, our study provided population-level trends in long-term competing mortality and survival, which provides useful insights for proper care for patients with HNC cancer.
In conclusion, competing non-cancer deaths remain frequent in HNC survivors, even long after treatment. Comorbidity-related deaths were distinctively predominant in the elderly, whereas intentional self-harm in the younger population. Population-specific surveillance and survivorship programs seem required. Awareness and a customized provision of care to the psychosocially vulnerable young and the frail elderly HNC survivors are warranted.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The study protocol was approved by the Institutional Review Board of the National Cancer Center (NCC2016-0041) of Korea. This study used secondary de-identified data. Written informed consents were waived. AcknowledgmentsThe authors would like to thank the staff of the Korea Central Cancer Registry and Statistics Korea.
This work was supported by the National Cancer Center of Korea (grant number NCC-1710300-3, NCC-2110450); and the National Research Foundation of Korea grant (grant number NRF-2020R1A-2C1A01011584, 2020R1A2C2005091) funded by the Korea Ministry of Science and ICT. The funding source had no role in the study design, data curation, or the analysis and interpretation of data.
Fig. 1Probabilities of survival, death from head and neck cancer and competing other causes by age group (yr). The probabilities of death were estimated by the cumulative incidence function. 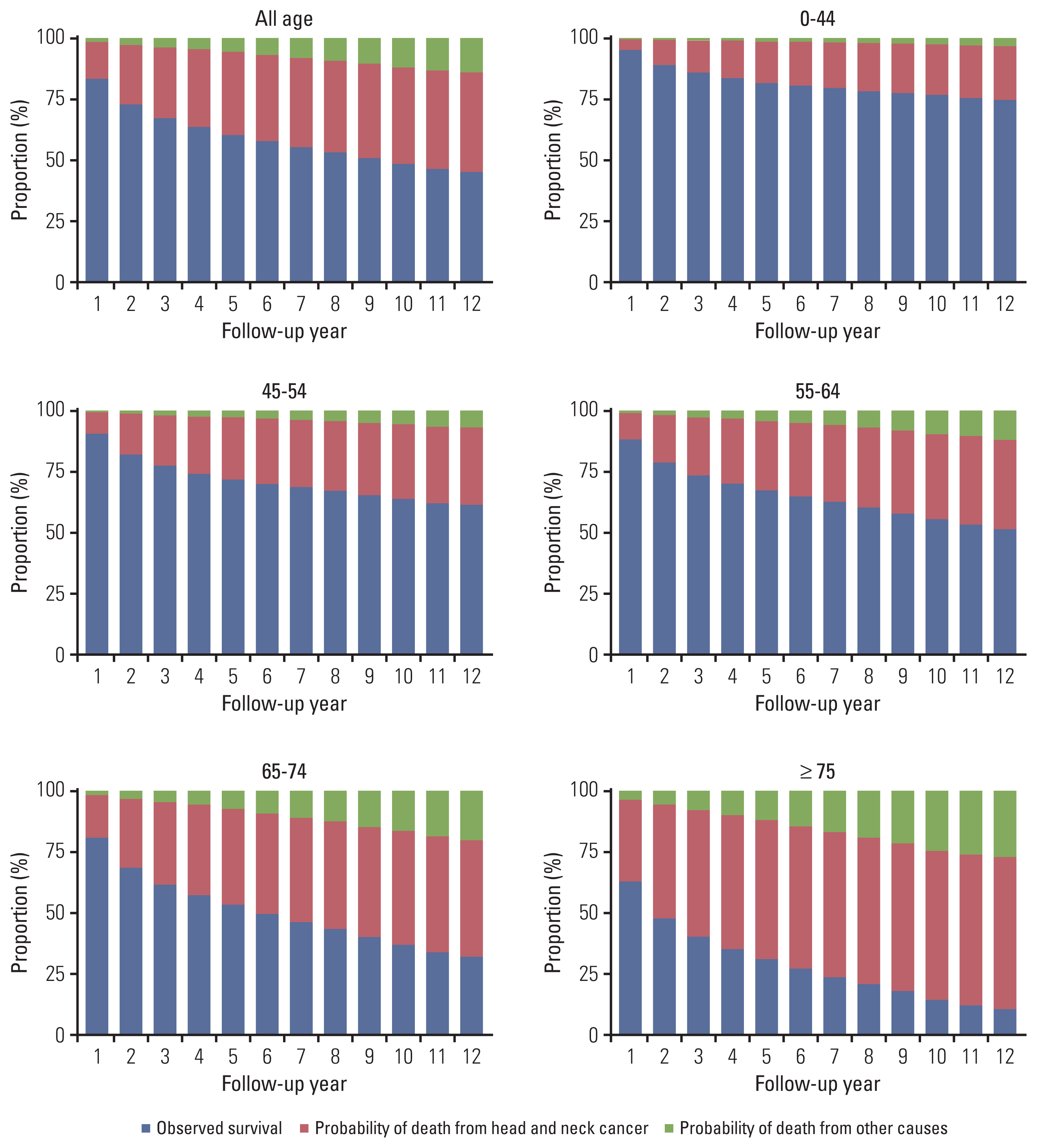
Fig. 2(A–C) Competing risks probabilities of death from the head and neck cancer and other causes by age and tumor stage. 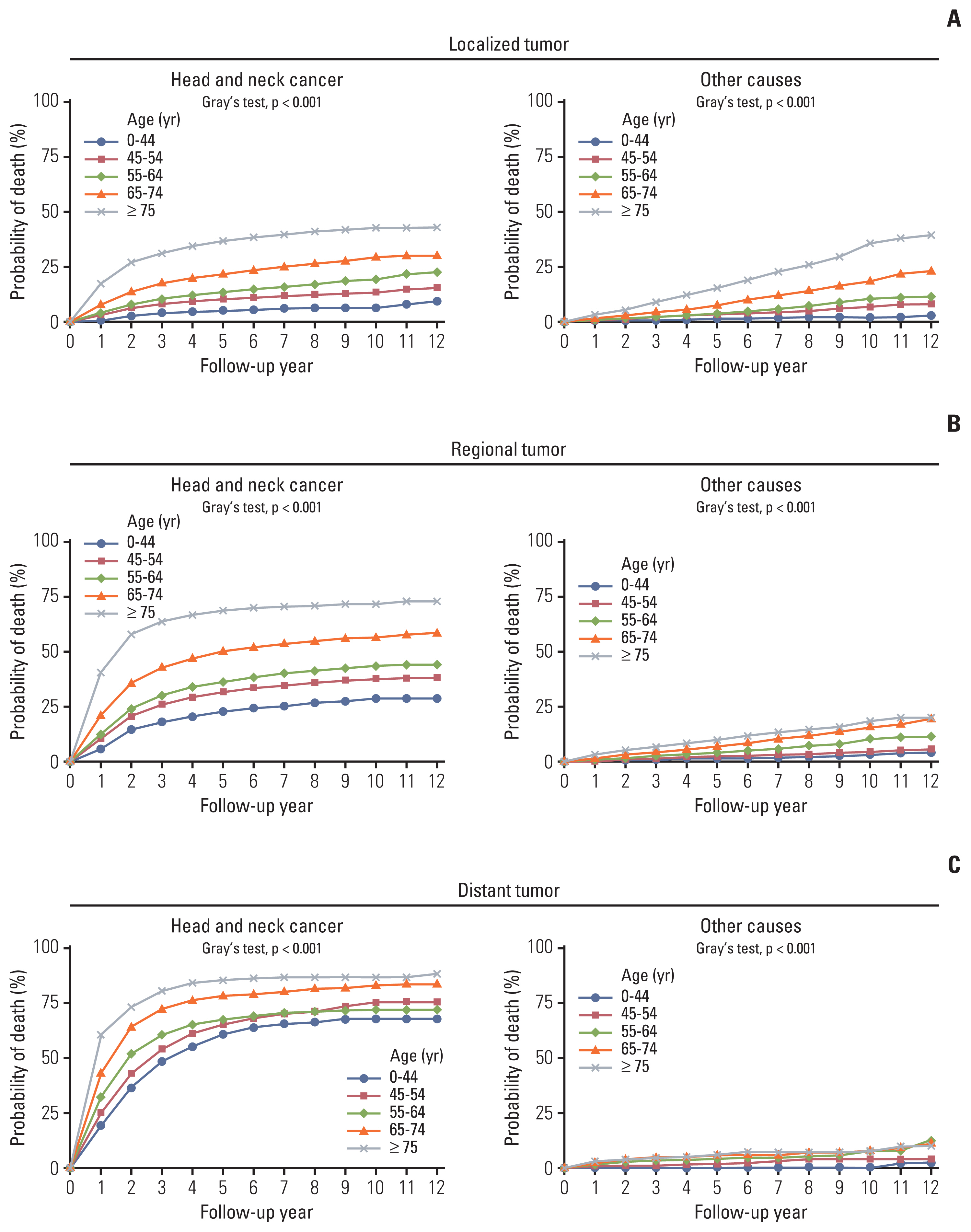
Table 1Patient characteristics and distribution of causes of death by sex, age group, and tumor stage, KCCR 2006–2016
Values are presented as number (%). Percentages are rounded to an integer. Patients diagnosed with primary head and neck cancer from January 1, 2006, to December 31, 2016, followed up to December 31, 2017. Results of patients whose tumor stage is unknown are not included. -, less than 10 cases; KCCR, Korea Central Cancer Registry. Table 2Standard mortality ratios of the most frequent non-cancer causes of death in cancer patients relative to the general population
Table 3Five and 10-year survival and probability of death from the head and neck cancer and competing other causes, KCCR 2006–2016
Table 4Risks of death due to head and neck cancer and other causes under the presence of competing risksa)
References1. Hall SF, Groome PA, Rothwell D. The impact of comorbidity on the survival of patients with squamous cell carcinoma of the head and neck. Head Neck. 2000;22:317–22.
2. Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120:1507–13.
3. Kim YJ, Kim JH. Increasing incidence and improving survival of oral tongue squamous cell carcinoma. Sci Rep. 2020;10:7877.
4. Nguyen NA, Ringash J. Head and neck cancer survivorship care: a review of the current guidelines and remaining unmet needs. Curr Treat Options Oncol. 2018;19:44.
5. Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010;15:994–1001.
6. Simpson MC, Massa ST, Boakye EA, Antisdel JL, Stamatakis KA, Varvares MA, et al. Primary cancer vs competing causes of death in survivors of head and neck cancer. JAMA Oncol. 2018;4:257–9.
7. Mell LK, Dignam JJ, Salama JK, Cohen EE, Polite BN, Dandekar V, et al. Predictors of competing mortality in advanced head and neck cancer. J Clin Oncol. 2010;28:15–20.
8. Massa ST, Osazuwa-Peters N, Christopher KM, Arnold LD, Schootman M, Walker RJ, et al. Competing causes of death in the head and neck cancer population. Oral Oncol. 2017;65:8–15.
9. Kim YH, Roh JL, Kim SB, Choi SH, Nam SY, Kim SY. Risk factors for competing non-cancer mortality after definitive treatment for advanced-stage head and neck cancer. Oral Dis. 2018;24:1217–25.
10. Shen W, Sakamoto N, Yang L. Cancer-specific mortality and competing mortality in patients with head and neck squamous cell carcinoma: a competing risk analysis. Ann Surg Oncol. 2015;22:264–71.
11. Vaisanen JA, Alho OP, Koivunen PT, Laara E. Cause-specific mortality in patients with head and neck cancer: long-term follow-up of a population-based cohort from 1986 to 2012 accounting for competing risks. Oral Oncol. 2018;79:20–6.
12. Gimeno-Hernandez J, Iglesias-Moreno MC, Gomez-Serrano M, Carricondo F, Gil-Loyzaga P, Poch-Broto J. The impact of comorbidity on the survival of patients with laryngeal squamous cell carcinoma. Acta Otolaryngol. 2011;131:840–6.
13. Piccirillo JF. Importance of comorbidity in head and neck cancer. Laryngoscope. 2000;110:593–602.
14. Paleri V, Wight RG, Davies GR. Impact of comorbidity on the outcome of laryngeal squamous cancer. Head Neck. 2003;25:1019–26.
15. Boje CR, Dalton SO, Primdahl H, Kristensen CA, Andersen E, Johansen J, et al. Evaluation of comorbidity in 9388 head and neck cancer patients: a national cohort study from the DAHANCA database. Radiother Oncol. 2014;110:91–7.
16. Hess CB, Rash DL, Daly ME, Farwell DG, Bishop J, Vaughan AT, et al. Competing causes of death and medical comorbidities among patients with human papillomavirus-positive vs human papillomavirus-negative oropharyngeal carcinoma and impact on adherence to radiotherapy. JAMA Otolaryngol Head Neck Surg. 2014;140:312–6.
17. Laara E, Korpi JT, Pitkanen H, Alho OP, Kantola S. Competing risks analysis of cause-specific mortality in patients with oral squamous cell carcinoma. Head Neck. 2017;39:56–62.
18. Forastiere AA, Weber RS, Trotti A. Organ preservation for advanced larynx cancer: issues and outcomes. J Clin Oncol. 2015;33:3262–8.
19. Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32:2735–43.
20. Lop J, Garcia J, Lopez M, Taberna M, Mena M, Alemany L, et al. Competing mortality in oropharyngeal carcinoma according to human papillomavirus status. Head Neck. 2019;41:1328–34.
21. Norregaard C, Gronhoj C, Jensen D, Friborg J, Andersen E, von Buchwald C. Cause-specific mortality in HPV+ and HPV− oropharyngeal cancer patients: insights from a population-based cohort. Cancer Med. 2018;7:87–94.
22. Choi JW, Park EC. Suicide risk after cancer diagnosis among older adults: a nationwide retrospective cohort study. J Geriatr Oncol. 2020;11:814–9.
23. Zaorsky NG, Zhang Y, Tuanquin L, Bluethmann SM, Park HS, Chinchilli VM. Suicide among cancer patients. Nat Commun. 2019;10:207.
24. Jung YS, Lim J, Jung KW, Ryu J, Won YJ. Metachronous second primary malignancies after head and neck cancer in a Korean cohort (1993–2010). PLoS One. 2015;10:e0134160.
25. Shin A, Jung YS, Jung KW, Kim K, Ryu J, Won YJ. Trends of human papillomavirus-related head and neck cancers in Korea: national cancer registry data. Laryngoscope. 2013;123:E30–7.
26. Kwon M, Roh JL, Song J, Lee SW, Kim SB, Choi SH, et al. Noncancer health events as a leading cause of competing mortality in advanced head and neck cancer. Ann Oncol. 2014;25:1208–14.
27. Takenaka Y, Yasui T, Enomoto K, Miyabe H, Morizane N, Ashida N, et al. Risk factors associated with competing mortality among patients with head and neck cancer in Japan. Acta Otolaryngol. 2016;136:325–9.
28. Jung KW, Won YJ, Kong HJ, Lee ES. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2016. Cancer Res Treat. 2019;51:417–30.
29. World Health Organization. International statistical classifi-cation of diseases and related health problems. Geneva: World Health Organization; 2004.
30. Shin HY, Lee JY, Song J, Lee S, Lee J, Lim B, et al. Cause-of-death statistics in the Republic of Korea, 2014. J Korean Med Assoc. 2016;59:221–32.
31. Cho H, Mariotto AB, Mann BS, Klabunde CN, Feuer EJ. Assessing non-cancer-related health status of US cancer patients: other-cause survival and comorbidity prevalence. Am J Epidemiol. 2013;178:339–49.
32. Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–98.
33. Kahn HA, Sempos CT. Statistical methods in epidemiology. New York: Oxford University Press; 1989.
34. Prentice RL, Kalbfleisch JD, Peterson AV Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–54.
35. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54.
36. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509.
37. Simpson WG, Klaassen Z, Jen RP, Hughes WM, Neal DE Jr, Terris MK. Analysis of suicide risk in patients with penile cancer and review of the literature. Clin Genitourin Cancer. 2018;16:e257–61.
38. Coatesworth AP, Tsikoudas A, MacLennan K. The cause of death in patients with head and neck squamous cell carcinoma. J Laryngol Otol. 2002;116:269–71.
39. Rose BS, Jeong JH, Nath SK, Lu SM, Mell LK. Population-based study of competing mortality in head and neck cancer. J Clin Oncol. 2011;29:3503–9.
40. Zapata I, Alvarez M, Hidalgo R, Pajares B, Garcia-Anaya MJ, Toledo MD, et al. Causes of death in patients with locally advanced head and neck cancer treated with radiotherapy and systemic therapy. BMC Cancer. 2019;19:1241.
41. Zaorsky NG, Churilla TM, Egleston BL, Fisher SG, Ridge JA, Horwitz EM, et al. Causes of death among cancer patients. Ann Oncol. 2017;28:400–7.
42. Pignon JP, le Maitre A, Maillard E, Bourhis J; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol. 2009;92:4–14.
43. Stoltzfus KC, Zhang Y, Sturgeon K, Sinoway LI, Trifiletti DM, Chinchilli VM, et al. Fatal heart disease among cancer pati-ents. Nat Commun. 2020;11:2011.
44. Zaorsky NG, Zhang Y, Tchelebi LT, Mackley HB, Chinchilli VM, Zacharia BE. Stroke among cancer patients. Nat Commun. 2019;10:5172.
45. Statistics Korea. Population projections for Korea: 2015–2065 (based on the 2015 population census). Daejeon: Statistics Korea; 2016.
46. Bank of Korea, Economic Research Institute. Population aging: impacts and policy imperatives. Seoul: Bank of Korea, Economic Research Institute; 2017.
47. Osazuwa-Peters N, Simpson MC, Zhao L, Boakye EA, Olomukoro SI, Deshields T, et al. Suicide risk among cancer survivors: head and neck versus other cancers. Cancer. 2018;124:4072–9.
48. Oh CM, Lee D, Kong HJ, Lee S, Won YJ, Jung KW, et al. Causes of death among cancer patients in the era of cancer survivorship in Korea: attention to the suicide and cardiovascular mortality. Cancer Med. 2020;9:1741–52.
49. World Health Organization. Suicide rates, age-standardized data by country. Geneva: World Health Organization; 2015.
50. Cain G. Why Koreans are killing themselves in droves [Internet]. Boston, MA: The World; 2014. [cited 2022 May 30]. Available from: https://theworld.org/stories/2014-03-13/why-koreans-are-killing-themselves-droves
51. Semple CJ, Sullivan K, Dunwoody L, Kernohan WG. Psychosocial interventions for patients with head and neck cancer: past, present, and future. Cancer Nurs. 2004;27:434–41.
52. Howren MB, Christensen AJ, Karnell LH, Funk GF. Psychological factors associated with head and neck cancer treatment and survivorship: evidence and opportunities for behavioral medicine. J Consult Clin Psychol. 2013;81:299–317.
53. Murphy BA, Deng J. Advances in supportive care for late effects of head and neck cancer. J Clin Oncol. 2015;33:3314–21.
54. Poorolajal J, Haghtalab T, Farhadi M, Darvishi N. Substance use disorder and risk of suicidal ideation, suicide attempt and suicide death: a meta-analysis. J Public Health (Oxf). 2016;38:e282–91.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||