AbstractPurposeThere has been accumulating evidence for the preventive effect of high physical activity on cancer. However, it is still unclear which level of physical activity is associated with the decreased risk of pancreatic cancer. The purpose of current study is to assess the association between the frequency of vigorous intensity physical activity and the risk of pancreatic cancer.
Materials and MethodsThe nationwide retrospective cohort study was conducted using the National Health Information Database. Study participants were 220,357 Koreans who received health check-up in 2009. They were divided into four groups by the weekly frequency of vigorous intensity physical activity longer than 20 minutes (group 1, no vigorous intensity physical activity (reference); group 2, 1–3 days; group 3, 4–5 days and group 4, 6–7 days). Cox proportional hazard model was used to calculate the adjusted hazard ratios (HRs) and 95% confidence interval (CI) for incident pancreatic cancer (adjusted HRs [95% CI]) according to the weekly frequency of vigorous intensity physical activity.
ResultsFor 4.38 years’ follow-up on average, 377 cases of pancreatic cancer developed. Subjects without incident pancreatic cancer had more favorable metabolic condition and higher physical activity than subjects with incident pancreatic cancer. Adjusted HRs and 95% CI indicated that only group 4 was significantly associated with the decreased risk of pancreatic cancer (group 1, reference; group 2, 1.10 [0.86–1.40]; group 3, 0.75 [0.45–1.25] and group 4, 0.47 [0.25–0.89]).
IntroductionPancreatic cancer is characterized by poor prognosis with dismal clinical course. Despite the development of diverse diagnostic and therapeutic approaches, overall 5-year survival rate is less than 6% [1], which ranks pancreatic cancer as 7th leading cause of cancer-related mortality worldwide [2]. Thus, it is clinically important to seek the way of preventing pancreatic cancer in terms of lowering medical burden of pancreatic cancer.
Evidence has indicated the role of metabolic derangement on the pathogenesis of pancreatic cancer. Epidemiologic studies have demonstrated that patients with obesity or type 2 diabetes mellitus (T2DM) had the relatively higher risk of pancreatic cancer, compared with their counterpart [3–6]. Insulin resistance is regarded as a potential pathologic mechanism for association between metabolic derangement and pancreatic cancer [7], where compensatory insulin hypersecretion, pancreatic cellular proliferation and elevated insulin-like growth factor-1 may contribute to the pathogenesis of pancreatic cancer [8,9].
It has been increasingly recognized that vigorous intensity physical activity benefits resolving metabolic abnormality. A prospective cohort study showed that vigorous physical activity was more strongly associated with the decreased risk of T2DM than other intensities-physical activity [10]. In a year-long randomized controlled trial of the effect of exercise on body weight and fat mass, moderate to vigorous intensity exercise led to the significant reduction of body weight, body mass index (BMI) and waist and hip circumferences in both men and women [11]. Thus, it is hypothesized that vigorous physical activity is potentially effective in reducing the risk of pancreatic cancer through improving metabolic condition. In practice, there was a report for the preventive effect of physical activity on pancreatic cancer in both cohort and case-control studies [12]. However, it is still early to draw the definite conclusion on association between physical activity and pancreatic cancer due to equivocal and heterogenous results [13,14]. Additionally, it seems that adopting wide range of physical activity including low to moderate level may be a challenge for ascertaining the risk of pancreatic cancer pertaining to the specific intensity of physical activity.
Using data of the National Health Insurance Corporation (NHIC) in Korea, we investigated the risk of incident pancreatic cancer according to the frequency of vigorous physical activity per week. The aim of study was to identify frequency of vigorous intensity physical activity related to decreased risk of pancreatic cancer.
Materials and Methods1. Data sourcesWe used the National Emergency Department Information System database from 2017 to 2019. In brief, thOur results were obtained from analyzing Korean statistics derived from the Korea National Health Information Database (NHID) operated by Korea NHIC that provides the National Health Insurance Service (NHIS) to Korean population. NHIS covers the entire population living in South Korea over 97%, suggesting that the NHID can represent the medical service usage of the entire Korean population [15]. In more detail, Korean medical institutions are mandatory to contract with NHIC, providing medical information of their healthcare users and patients to NHID run by NHIC. Therefore, the NHID is a public database containing the medical information and socio-demographic variables for Korean population collected from health care utilization and health check-up.
2. Study participantsInitial number of study participants was 223,551 Koreans who received health check-up in 2009. Their medical information and disease histories were recorded in the NHID. We reviewed their medical information from 2002 to date before health check-up in 2009, and excluded participants relevant to exclusion criteria as follows: present past history of pancreatic cancer based on international code of disease (ICD-C25 [C25.0–25.9]), and missing data for physical activity. Among the 223,355 participants, 194 subjects with history of pancreatic cancer and 3,000 subjects with missing data for physical activity were excluded from study participants We further excluded two subjects with both past history of pancreatic cancer and missing data for physical activity from study participants. Finally, the total number of study participants was 220,357 (Fig. 1). The total follow-up period was 965,829.1 person-year and average follow-up period was 4.38 (standard deviation, 0.45) person-year.
3. Health survey examinations and laboratory measurementsThis study was based on analyzing the data derived from health check-up performed in 2009. Koreans over age of 40 years are required to receive health check-up served by NHIS annually or biennially in medical institutions contracting with NHIC. The aim of health check-up is early detection of chronic illness and promoting the general health in Korean population.
The health check-up is conducted thorough two stages. The first stage is an interview between subjects and healthcare provider to discover symptoms or abnormalities. Interview is a screening to find out the possibility or risk of specific disease. The second stage is more detailed examinations to confirm the presence of disease. Detailed examinations are composed of self-questionnaire assessing lifestyle, health behavior and past medical histories, anthropometric measurements, and laboratory examinations.
Smoking amount was defined as pack-year. The pack-year was calculated from the smoking related questionnaire. Subjects with alcohol intake more than three times a week were regarded as alcohol drinker. BMI was calculated as weight (kg) divided by the square of height (m). Waist circumference (WC) was measured at the midpoint between the bottom of the rib cage and top of the iliac crest. Systolic blood pressure (BP) and diastolic BP were measured by trained examiners. The following laboratory data were measured at health check-up and used as baseline clinical characteristics: fasting blood glucose, total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, serum creatinine (SCr), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ-glutamyltransferase (GGT). Kidney function was measured with estimated glomerular filtration rate (eGFR), which was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation: eGFR=141×min(SCr/K, 1)a×max(SCr/K, 1)−1.209×0.993age×1.018 [if female] ×1.159 [if Black], where SCr is serum creatinine, K is 0.7 for females and 0.9 for males, a is −0.329 for females and −0.411 for males, min indicates the minimum of SCr/K or 1 and max indicates the maximum of SCr/K or 1 [16].
4. Assessment of physical activityInternational Physical Activity Questionnaire (IPAQ) short form was used in assessing the degree of physical activity. The detailed question was as follows; “During the last 7 days, how many days and how long (minutes) did you do vigorous physical activities like heavy lifting, digging, aerobics, or fast bicycling?”, “During the last 7 days, on how many days and how long (minutes) did you do moderate physical activities like carrying light loads, bicycling at a regular pace, or doubles tennis?”, “During the last 7 days, on how many days and how long (minutes) did you walk ?”, During the last 7 days, how much time (hour) did you spend sitting on a day?”. Subjects were divided into four groups according to the frequency of days per week that they performed vigorous intensity physical activities at least 20 minutes as follows: group 1, 0 day per week; group 2, 1–3 days per week; group 3, 4–5 days per week and group 4, 6–7 days per week. Metabolic equivalent of task (MET) was calculated as follows (minutes/week): walking MET (3.3×walking minutes×walking days per week)+moderate MET (4.0×moderate-intensity activity minutes×moderate days per week)+vigorous MET (8.0×vigorous-intensity activity minutes×vigorous-intensity days per week) [17].
5. Outcome definitionsThe identification of incident pancreatic cancer was based on reviewing NHID linked to the department of Statistics Korea in NHIC. Korean medical institutions contracting with NHIC are mandatory to provide the medical information of patients to NHID. For example, if pancreatic cancer is detected in asymptomatic or symptomatic patients by imaging modalities or surgical operation, medical institutions should register patients with newly identified pancreatic cancer into NHID as pancreatic cancer with ICD-code (C25 [C25.0–25.9]). Our study was based on NHID, and thus, we identified the incidence of pancreatic cancer on the basis of ICD-code (C25 [C25.0–25.9]) registered in NHID. Reviewing NHID before and at the time of receiving health check-up (2009), we firstly excluded all of individuals with previously registered ICD-C25 (C25.0–25.9), and enrolled individuals without previously registered ICD-C25 (C25.0–25.9) into study subjects. Through reviewing NHID from date after a time of receiving medical health check-up in 2009 to December 31th in 2013, we identified subjects with newly registered ICD-C25 (C25.0–25.9), and enlisted them into incident cases of pancreatic cancer. Therefore, the follow-up period of subjects with incident pancreatic cancer was from the date on health check-up (2009) to date on diagnosis of pancreatic cancer.
6. Statistical analysisData were expressed as means±standard deviation for continuous variables and percentages of the number for categorical variables. The one-way ANOVA and chi-square test were used to analyze the statistical differences among the characteristics of the study participants at the time of enrollment in relation to the four groups of baseline physical activity levels. The person-years were calculated as the sum of follow-up times from the baseline until the diagnosis time of pancreatic cancer development or until December 31, 2013. To evaluate the associations of baseline physical activity levels and incident pancreatic cancer, we used Cox proportional hazards models to estimate adjusted hazard ratios (HRs) and 95% confidence intervals (CI) for incident pancreatic cancer. Additionally, the Cox proportional hazard models were used in analyzing the risk of pancreatic cancer according to the quartile levels of MET.
The Cox proportional hazard models were adjusted for the multiple confounding factors. In the multivariate models, we included variables that might confound the relationship between physical activity and incident pancreatic cancer, which include: age, sex, BMI, systolic BP, fasting blood glucose, LDL-cholesterol, eGFR, GGT, smoking amount (pack-year), and alcohol intake. To test the validity of the Cox proportional hazard models, we checked the proportional hazard assumption. The proportional hazard assumption was assessed by log-minus-log survival function and found to be graphically unviolated. A likelihood ratio test was used to test whether there were positive linear dose-response relationships of the HRs for pancreatic cancer with increasing the frequency of vigorous physical activity. p-values < 0.05 were considered to be statistically significant. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Cary, NC).
ResultsDuring 965,829.1 person-years of follow-up, 377 (0.17%) cases of incident pancreatic cancer developed between 2009 and 2013.
Table 1 presents the baseline characteristics of study participants according to the frequency of vigorous intensity physical activity. Our study participants were of the elderly (overall mean age, 58.1±8.8) with a preponderance of men. Group 4 (6–7 vigorous intensity physical activity per week) had the higher mean values in fasting glucose, BP, BMI, WC and the proportion of alcohol drinker than other groups. However, TC, TG, and LDL-cholesterol were relatively low in group 4. With respect to physical activity, MET increased with the frequency of vigorous intensity physical activity, but sitting time was not significantly different among groups.
Compared with group without incident pancreatic cancer, group with incident pancreatic cancer tended to have the more adverse clinical characteristics in age, systolic BP, TG, HDL-cholesterol, fasting glucose, eGFR, AST, ALT, and GGT (Table 2). Parameters showing the significant difference between two groups were illustrated in Fig. 2. Additionally, group with incident pancreatic cancer had the poorer health behavior (smoking amount and proportion of alcohol drinker) and lower physical activity in MET and moderate intensity physical activity than group without incident pancreatic cancer.
Table 3 indicates the HRs and 95% CI for pancreatic cancer according to the frequency of vigorous intensity physical activity. In unadjusted model, the HRs and 95% CI for pancreatic cancer delineated the inverse relationship between frequency of vigorous intensity physical activity and risk of incident pancreatic cancer (group 1, reference; group 2, 0.85 [0.68–1.07]; group 3, 0.60 [0.36–0.99] and group 4, 0.51 [0.27–0.95], respectively) (p for trend=0.014). The statistical significance of association was markedly attenuated after adjustment for multiple covariates, but group 4 showed the maintained statistical significance (group 1, reference; group 2, 1.10 [0.86–1.40]; group 3, 0.75 [0.45–1.25] and group 4, 0.47 [0.25–0.89], respectively) (p for trend=0.004).
Out of 377 subjects with incident pancreatic cancer, 22 subjects developed pancreatic cancer within 6 months from the date of cohort participation. Even in an analysis excluding such 22 subjects from study participants (S1 Table), group 4 was significantly associated with decreased risk of pancreatic cancer, compared with group 1, reference; group 2, 1.12 [0.87–1.44]; group 3, 0.81 [0.49–1.35] and group 4, 0.51 [0.37–0.96], respectively) (p for trend=0.009).
S2 Table presents the risk of pancreatic cancer according to the quartile levels of MET. Unadjusted analysis showed that the third and fourth quartile group of MET had the lower risk of pancreatic cancer than the first quartile group. However, in multivariate adjusted analysis, any quartile groups didn’t show the significant association with decreased risk of pancreatic cancer compared with the first quartile group of MET (first quartile, reference; second quartile, 1.14 [0.82–1.39]; third quartile, 0.90 [0.73–1.16] and fourth quartile, 0.84 [0.65–1.04], respectively) (p for trend=0.005).
DiscussionIt is well established that physical activity has the favorable effect on metabolic health. Physical activity is effective in controlling body weight and ameliorating insulin sensitivity [18,19]. Published studies have suggested that elevated physical activity lessens chronic inflammation, oxidative stress and DNA damage [20,21]. These results link to a notion that elevated physical activity may reduce cancer risk. In practice, previous literatures have reported the potential benefit of increased physical activity in preventing cancer [22–24]. However, evidence is still insufficient in corroborating the preventive effect of physical activity on pancreatic cancer. In particular, it is less clear as to which level of physical activity contributes to preventing pancreatic cancer. Difficulty in assessing the intensity of physical activity may be a reason for ambiguous results. We took note of a notion that vigorous intensity physical activity is potentially more powerful than other intensities of physical activity in preventing metabolic disorders. Simplifying the level of physical activity into vigorous intensity, we evaluated the risk of incident pancreatic cancer according to the frequency of vigorous intensity physical activity.
In the present study, compared group 1 (individuals not conducting vigorous physical activity), group 4 (individuals conducting vigorous physical activity for 6–7 days per week) showed the 53% decreased risk of incident pancreatic cancer (0.47 [0.25–0.89]). Compared with the previous reports, it seems that more than 50% reduction of pancreatic cancer is relatively high. Pooled estimates indicated reduction in pancreatic cancer risk with higher levels of total (0.72 [0.52–0.99]) and occupational activity (0.75 [0.59–0.96]) [13]. Additionally, high physical activity is associated with decreased risk of pancreatic cancer by 7% in cohort study and 22% in case-control study [12]. As a plausible explanation for this finding, it is considered that only vigorous intensity physical activity was used as independent variable. We assessed the risk of pancreatic cancer according to frequency of only vigorous intensity physical activity, and only a few subjects (4.71%) were relevant to category with 6–7 times of vigorous intensity physical activity. In our point of view, 6–7 times of vigorous intensity physical activity per week seems to be considerably intensified physical activity. Additionally, group 4 maintained the intensified physical activity for relatively long duration (27.6±10.3). Thus, the preventive effect of physical activity might be greatly manifested in group 4. Recent study for Chinese adult also showed that meeting the recommended minimum exercise threshold was associated with 41% decreased risk of pancreatic cancer risk (HR, 0.59; 95% CI, 0.40 to 0.87) [25]. Taken together with these reports, it is postulated that daily vigorous intensity physical activity has a magnificient preventive effect on pancreatic cancer.
The lifestyle factors like physical activity and sedentary behavior have been studied for their associations with cancer. Physical activity is inversely associated with and sedentary behavior is positively associated with an increased risk of more than ten types of cancer including colorectal and breast cancer [26]. In our result, groups 1 and 2 accounted for most cases (93%) of incident pancreatic cancer, and adjusted analysis indicates that group 1 had the higher risk for pancreatic cancer than group 4. Total MET was lower in group with incident pancreatic cancer (858±345) than group without incident pancreatic cancer (874±378). These findings suggest that physical inactivity is potentially associated with the increased risk of pancreatic cancer. Nonetheless, our results can’t assure that physical inactivity is a definite risk factor for pancreatic cancer. Although group 4 had the lower risk for pancreatic cancer than lowest physical activity group 1, group 2, and group 3 didn’t show the significantly decreased risk of pancreatic cancer, compared with group 1. Additionally, sitting time was not significantly different between group with and without incident pancreatic cancer. A previous study demonstrated that physical inactivity was not risk factors for pancreatic cancer [27]. Therefore, next step for research should analyze the association between comprehensive levels of physical activity and the risk of pancreatic cancer.
In our analysis, age, fasting glucose, smoking, and male sex were significantly associated with the increased risk of pancreatic cancer. This result is corresponding to previous reports suggesting the metabolic risk factors for pancreatic cancer [28,29]. Nonetheless, group 4 was not characterized by the favorable baseline metabolic profiles. Group 4 had the higher mean age (60.1±8.5 years) than other groups. The elderly men with retirement may have elevated interest on health, resulting in the increased physical activity. Therefore, it is perplexing that group 4 had the higher levels in smoking amount and proportion of drinker than other groups. This finding may be partly attributable to the cultural feature in Korean that smoking and alcohol consumption are more generous and prevalent in the elderly men. Additionally, exercise or leisure sports tend to be performed in club or community activity, which frequently accompanies alcohol drinking. These factors may be responsible for the higher smoking and alcohol intake in group 4. The relatively older age in group 4 may contribute to their higher levels in BP, fasting glucose, BMI, and WC than those in other groups. However, in group 4, mean levels of BP, BMI, and WC were within normal range, and mean fasting glucose level didn’t show the remarkable difference among groups (100.8±25.4 to 102.0±24.8 mg/dL). Moreover, group 4 had the relatively low levels in mean TC, TG, and LDL-cholesterol. Thus, it seems that elevated physical activity had a favorable effect on metabolic condition.
Men were predominant in our study participants. The accessibility of men to health check-up may be a plausible explanation for the preponderance of men in our study participants. Although health check-up is accessible to all of Koreans over age of 40, socially active persons tend to receive health check-up. Because men are more socially active than women in Korea society, men are more likely to receive health check-up than women.
Limitations of current study should be recognized. First, our raw data didn’t include detailed information about pancreatic cancer and medical usage of study subjects. Therefore, we are limited in identifying the stage and the pathology type of incident pancreatic cancer. Additionally, we are not accessible to medical usage of subjects with incident pancreatic cancer. Second, determination of incident pancreatic cancer was based only on ICD-code in NHID. Thus, there is a possibility that incidence of pancreatic cancer in our study is different from that in other Korean cancer statistics. Using other cancer statistics may allow us to obtain the more accurate incidence. However, we couldn’t match our study subjects with those in other cancer statistics because personal information of study subjects is absent in our raw data. Thus, it is impossible to use other cancer statistics in determining incident pancreatic cancer. Third, since assessment of physical activity was based on IPAQ short form, our results didn’t reflect the broad spectrum of physical activities covered by working, house and yard working, getting from place to place, in spare time for recreation and exercise or sport. Fourth, the follow-up period for pancreatic cancer was relatively short (4.38 person-years). Short follow-up period may link to the relatively small cases of incident pancreatic cancer. Therefore, it is inferred that longer follow-up period results in the more substantial results with the higher incidence of pancreatic cancer.
Near daily vigorous intensity physical activity longer than 20 minutes was significantly associated with the decreased risk of pancreatic cancer. These results suggest the preventive effect of daily vigorous intensity physical activity on pancreatic cancer.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement Ethics approvals for the study protocol and analysis of the data were obtained from the institutional review board of Kyung Hee University Hospital (No. 2018-12-020). The informed consent requirement was exempted by the institutional review board because researchers only accessed retrospectively a de-identified database for analysis purposes. AcknowledgmentsWe used the National Health Insurance Service–National Sample Cohort database and the dataset was obtained from the National Health Insurance Service. Our study findings were not related to the National Health Insurance Service.
This work was supported by the National Research Foundation of Korea in 2020 (grant number: 2020R1G1A1102257). The funding organization had no role in the design or conduct of this research.
We used the National Health Insurance Service–National Sample Cohort database and the dataset was obtained from the National Health Insurance Service. Our study findings were not related to the National Health Insurance Service.
Fig. 2Parameters showing statistically significant difference between group without incident pancreatic cancer (control group) and group with incident pancreatic cancer (pancreatic cancer group). AST, aspartate aminotransferase; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyltransferase; HDL, high-density lipoprotein; WC, waist circumference. 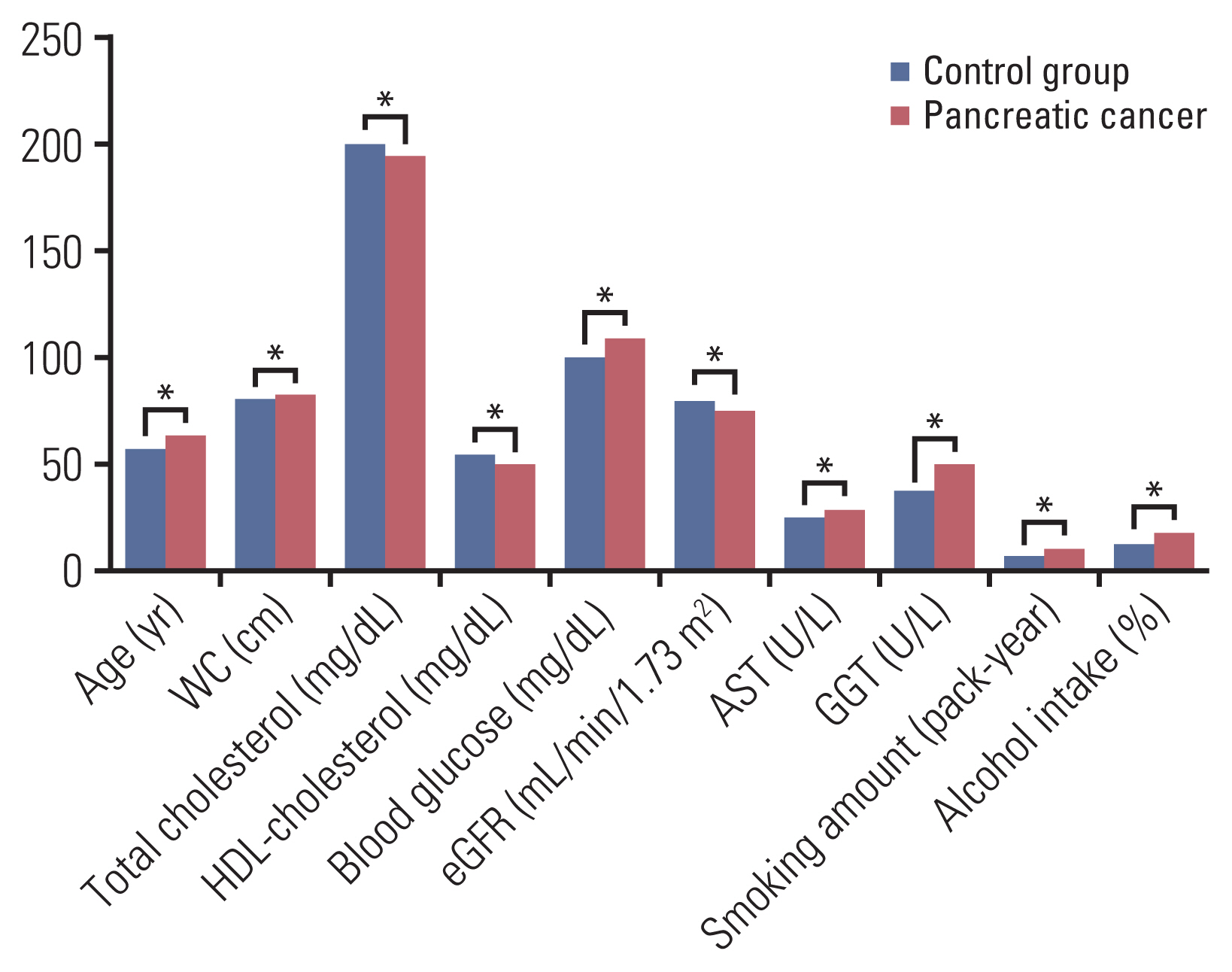
Table 1Baseline characteristics of participants according to the four groups of the physical activity levels (n=220,357)
Values are presented as mean±standard deviation or number (%). Group 1, performing 0 day per week; group 2, performing 1-3 days per week; group 3, performing 4-5 days per week; group 4, performing 6-7 days per week. ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyltransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MET, metabolic equivalent of task; PA, physical activity; SCr, serum creatinine; WC, waist circumference. Table 2Comparison between participants with and without incident pancreatic cancer
Values are presented as mean±standard deviation or number (%). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyltransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; MET, metabolic equivalent of task; PA, physical activity; SCr, serum creatinine; WC, waist circumference. Table 3Hazard ratios and 95% confidence intervals for the incidence of pancreatic cancer according to the four groups divided by the frequency of vigorous physical activity Incidence density: number of incident pancreatic cancer per 10,000 person-year. Adjusted model: multivariate adjustment for age, sex, BMI, systolic BP, fasting blood glucose, LDL-cholesterol, eGFR, GGT, smoking amount (pack-year), and alcohol intake. Group 1, performing 0 day per week; group 2, performing 1-3 days per week; group 3, performing 4-5 days per week; group 4, performing 6-7 days per week. BMI, body mass index; BP, blood pressure; eGFR, estimated glomerular filtration rate; GGT, γ-glutamyltransferase; LDL, lowdensity lipoprotein. References2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
3. Arslan AA, Helzlsouer KJ, Kooperberg C, Shu XO, Steplowski E, Bueno-de-Mesquita HB, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med. 2010;170:791–802.
4. Jiao L, Berrington de Gonzalez A, Hartge P, Pfeiffer RM, Park Y, Freedman DM, et al. Body mass index, effect modifiers, and risk of pancreatic cancer: a pooled study of seven prospective cohorts. Cancer Causes Control. 2010;21:1305–14.
5. Everhart J, Wright D. Diabetes mellitus as a risk factor for pancreatic cancer: a meta-analysis. JAMA. 1995;273:1605–9.
6. Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–83.
7. Cowey S, Hardy RW. The metabolic syndrome: a high-risk state for cancer? Am J Pathol. 2006;169:1505–22.
8. Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–8.
10. Hu FB, Sigal RJ, Rich-Edwards JW, Colditz GA, Solomon CG, Willett WC, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA. 1999;282:1433–9.
11. McTiernan A, Sorensen B, Irwin ML, Morgan A, Yasui Y, Rudolph RE, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring). 2007;15:1496–512.
12. Behrens G, Jochem C, Schmid D, Keimling M, Ricci C, Leitzmann MF. Physical activity and risk of pancreatic cancer: a systematic review and meta-analysis. Eur J Epidemiol. 2015;30:279–98.
13. O’Rorke MA, Cantwell MM, Cardwell CR, Mulholland HG, Murray LJ. Can physical activity modulate pancreatic cancer risk? a systematic review and meta-analysis. Int J Cancer. 2010;126:2957–68.
14. Bao Y, Michaud DS. Physical activity and pancreatic cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev. 2008;17:2671–82.
15. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: the National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017;46:e15.
16. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12.
17. Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ) [Internet]. International Physical Activity Questionnaire; 2020. [cited 2020 Dec 10]. Available from: http://www.ipaq.ki.se
18. Mann S, Beedie C, Balducci S, Zanuso S, Allgrove J, Bertiato F, et al. Changes in insulin sensitivity in response to different modalities of exercise: a review of the evidence. Diabetes Metab Res Rev. 2014;30:257–68.
19. Barte JC, Veldwijk J, Teixeira PJ, Sacks FM, Bemelmans WJ. Differences in weight loss across different BMI classes: a meta-analysis of the effects of interventions with diet and exercise. Int J Behav Med. 2014;21:784–93.
20. Cash SW, Beresford SA, Vaughan TL, Heagerty PJ, Bernstein L, White E, et al. Recent physical activity in relation to DNA damage and repair using the comet assay. J Phys Act Health. 2014;11:770–6.
21. Herder C, Peltonen M, Koenig W, Sutfels K, Lindstrom J, Martin S, et al. Anti-inflammatory effect of lifestyle changes in the Finnish Diabetes Prevention Study. Diabetologia. 2009;52:433–42.
22. World Cancer Research Fund, American Institute for Cancer ResearchFood, nutrition, physical activity and the prevention of cancer: a global perspective. Washington, DC: American Institute for Cancer Research; 2007.
23. Kyu HH, Bachman VF, Alexander LT, Mumford JE, Afshin A, Estep K, et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ. 2016;354:i3857.
24. Matthews CE, Moore SC, Arem H, Cook MB, Trabert B, Hakansson N, et al. Amount and intensity of leisure-time physical activity and lower cancer risk. J Clin Oncol. 2020;38:686–97.
25. Wu L, Zheng W, Xiang YB, Gao YT, Li HL, Cai H, et al. Physical activity and pancreatic cancer risk among urban Chinese: results from two prospective cohort studies. Cancer Epidemiol Biomarkers Prev. 2018;27:479–87.
26. Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol. 2017;18:e457–71.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||