AbstractPurposeColorectal cancer (CRC) is increasing in South Korea due to westernized eating habits and regular health check-ups. The Korean Health Insurance Review and Assessment Service (HIRA) has conducted a national quality assessment of the treatment of CRC. This study examined the quality assessment report of the Korean HIRA and analyzed the status of practice pattern and the epidemiology of CRC in South Korea.
Materials and MethodsThe number of subjects was determined based on the number of surgical procedures in each institution during 2012–2017. The institution types were classified according to the number of beds and the composition of oncologic specialists. Twenty-one indicators for diagnosis, chemotherapy, radiotherapy, surgery, pathology, and mortality were analyzed and the inter-institutional variation for each indicator was calculated.
ResultsAmong 21 evaluation indices, indicators related to medical records, receipt of chemotherapy with a high coefficient of variation of ≥ 0.1% were improved over 6 years until the survey in 2017. In the analysis of indices affecting surgical mortality, the regional lymph node resection and examination rate (p=0.022) showed a negative correlation with surgical mortality. Hospitalization stay (p < 0.001) and hospitalization cost (p=0.002) were positively correlated with surgical mortality.
ConclusionThis study showed that the treatment quality and examination status for CRC in South Korea were appropriate for improving relevant medical records, receipt of chemotherapy, maintaining the quality of treatment, and mortality. These analyses could be the basis for developing an improved quality assessment program worldwide.
IntroductionColorectal cancer (CRC) is the second most common cancer in South Korea and has the highest growth rate among the top five cancers [1]. The incidence of CRC is increasing in Asian countries due to westernized lifestyles and regular health check-ups. CRC is the third-highest cause of death from cancer, and death from CRC is steadily increasing in South Korea [2].
Thus, the Korean Health Insurance Review and Assessment Service (HIRA) has conducted a quality assessment of the four major cancers to minimize the variation in medical treatments among medical institutions and improve the quality of cancer treatment practices. Since 2011, a quality assessment of CRC has been performed on patients with primary CRC who underwent surgery in all Korean institutions. Twenty-one evaluation indicators were selected and the value and improvement rate of each indicator according to the institution were analyzed to calculate the grade, which was reported annually by the HIRA.
The trend in 21 indicators has been assessed over the past 6 years. This study evaluated the data of the quality assessment and analyzed the status of the treatment of CRC in South Korea and the relationship between mortality, medical cost, and the indicators.
Materials and Methods1. Data sourceFrom January 2011 to December 2016, inpatient health insurance, medical benefits, billing statements, medical records, and mortality data from the Ministry of the Interior and Safety were collected annually according to the institution where patients received colorectal surgery. The subjects were included as follows: (1) patients aged 18 years or older who had surgery for primary CRC; (2) Korean Standard Disease Classifications C18–C20 (including major and minor diseases), which indicate colon and rectal cancer; (3) those undergoing colectomy, rectal, and sigmoid colon resection, total colectomy or total mesorectal resection; and (4) cancer stage American Joint Committee on Cancer (AJCC) I–IV (AJCC I–III as a process indicator). Patients who received prior chemotherapy or radiation therapy were also included in this study. Patients who were diagnosed with recurrent or secondary cancer, patients who were transferred after surgery at other institutions, patients with stage 0, and patients with other primary cancers within five years were excluded from the analysis.
2. Methods and process of assessmentThe study population was selected using billing statements and evaluation data were collected using a web-based data collection system. To increase the accuracy of the survey data, sampling of the cases and verification of the medical records were conducted. The institution types were classified into advanced general hospitals, general hospitals, hospitals, and clinics according to the number of beds and the composition of oncologic specialists. The definition of each evaluation index is summarized in Fig. 1. A total of 21 indicators were analyzed, which consisted of one structural indicator, which presents the workforce in the institution before treatment; 17 process indicators that could be evaluated during the treatment process; and three outcome indicators that extracted socio-economic costs and surgical mortality data after treatment.
The number of subjects to be surveyed was determined based on the number of surgical procedures. Surveys for treatment-related index (process index) were conducted in all institutions. Surveys for the cost and mortality (result index) were conducted in institutions with more than more than 150 operations a year. Surveys of the outcome index were scored in total. In the assessment, the results for each evaluation index and institution were calculated, and the overall scores for each medical institution were evaluated by grading the evaluation indicators. Fig. 2 outlines the assessment process.
3. Statistical analysisThe difference and trend in the institutional variation for each indicator for each year were calculated. Inter-institutional variation was analyzed using the variation coefficient. Index analysis related to surgical mortality was performed, and the surgical mortality rate and each index were extracted for each organ type. Process indices with a significant effect were analyzed by linear regression. SPSS ver. 24 (IBM Corp., Armonk, NY) was used for the statistical analysis.
Results1. Study populationFrom 2012 to 2017, the assessment data of CRC were analyzed every year. The study population was categorized by the type of institution, surgical procedure, type of surgery, and type of cancer (colon or rectum), sex, age, and cancer stage.
In the current status by institutional type, the number of advanced general hospitals, general hospitals, and clinics maintained levels similar to previous years and the number of hospitals continued to decrease, showing a 52% decrease from 50 in 2013 to 24 in 2017. According to the number of surgeries, medical institutions with fewer than nine cases decreased, while the number of institutions with 10 or more operations remained similar during the survey period. The average number of rectal and sigmoid colon resections, colectomies, total colectomies, or total mesorectal resections was 11,174, 6,551, and 49 cases, respectively, and also remained similar throughout the study period. The average number of patients with colon and rectal cancer was 14,153 and 3,752, and male and female patients were 10,694 and 7,212, respectively, with 48% more cases in men. The age distribution remained similar throughout the study period, but 80-year-olds and older showed a 53% increase from 1,437 cases in 2013 to 2,200 cases in 2017. According to the cancer stage, the average number of patients with stage 1 to 4 was 3,829, 5,144, 6,443, and 2,439, respectively, and the number of stage 3 was the highest among them. Table 1 summarizes the yearly trends and average values for the study population.
2. Results of quality assessmentThe annual inter-institutional variation coefficient was calculated in 21 evaluation indices. The coefficient of variation is the ratio of the standard deviation to the mean, and the higher the value, the greater the inter-organ variation. The trend in the coefficient of variation for each indicator is illustrated in Fig. 3.
Among the 21 evaluation indices, the indicators in 2012 with a high coefficient of variation of ≥ 0.1% were the composition of professional personnel, the performance rate of preoperative pain evaluation, cancer family history-taking, preoperative further work-up, cancer stage records, the number of patients who did not receive chemotherapy in stage I, adjuvant chemotherapy within eight weeks after surgery in stage II–III, recommended adjuvant chemotherapeutic regimen, flow sheet use, postoperative radiotherapy, the average hospital stay, average treatment cost, and surgical mortality. Of the 13 factors with high coefficients of variation, the coefficient of variation decreased to more than 80% in the preoperative pain assessment rate, cancer family history-taking, cancer stage records, no chemotherapy in stage I, adjuvant chemotherapy within eight weeks after surgery, flow sheet use, and recommended adjuvant chemotherapeutic regimen rates in stage II–III when evaluated in 2017. In contrast, the indicators for which the coefficient of variation was not significantly reduced to more than 80% were the composition of professional personnel, preoperative further work-up, radiotherapy after surgery, the average hospital stay, treatment costs, and surgical mortality. These six indicators did not significantly reduce inter-organ variation compared to the other indicators.
In the first evaluation, the indicators for which the inter-institutional variation was not large with coefficients of variation less than 0.1% were records of resection completeness, carcinoembryonic antigen testing within three months after surgery, pathology report records, regional lymph node dissection and examination, training on stoma management, explanation of chemotherapy, prescriptions for antiemetics, and concurrent chemoradiation for rectal cancer.
3. Analyses of indicators related to surgical mortalityTo analyze the evaluation index related to the operative mortality rate, a linear regression analysis was performed between the operative mortality rate by institution type and 20 evaluation indices each year. Among these, there was a negative correlation between the regional lymph node resection and examination rate and the surgical mortality rate (p=0.022). In addition, there was a significant positive correlation between the average hospitalization stay (p < 0.001) and the average hospitalization cost (p=0.002). The other 17 indicators showed no significant correlations. The linear regression analysis graph for three significant indicators is shown in Fig. 4.
DiscussionCRC has the third-highest cancer incidence and mortality in Korea, and its survival rate has been rising steadily since 2010 [1]. However, due to the high incidence, it is necessary to improve the socio-economic cost and tumor control for CRC. The standard treatment for CRC without distant metastasis is surgery, and chemotherapy or radiotherapy has been performed with neoadjuvant or adjuvant aims, depending on the stage and pathologic findings after surgery for rectal cancer [3–5]. In the European Consensus Conference Colon and Rectum with this multimodality treatment method, treatment protocols differed depending on the institution and in examination procedures for diagnosis and staging [6]. One of the European Union’s activities for cancer called the European Partnership for Action Against Cancer (EPAAC) is working to reduce the variation between healthcare services within and between countries [7]. EUROCARE-5 analyzed the cancer survival rates in 29 European countries between 1999 and 2007 and reported that the differences in survival rates for CRC and lymphoma were large [8]. This has led to the need for quality frameworks and a commitment to high-quality patient-oriented care.
Since 2011, a government agency called HIRA in Korea has implemented an annual national quality assessment program to evaluate the treatment of CRC. This is expected to be an important part of national cancer management to reduce variations in social costs and improve oncological outcomes. In an analysis of the evaluation indices in 2012, the surgery-related indices showed relatively little variation between organs. In addition, there were relatively small differences according to institution types in chemotherapy, antiemetic prescriptions, and concurrent chemoradiation for rectal cancer. However, the indications, timing of treatment initiation, and chemotherapy protocols were largely different for each institution. Furthermore, pretreatment history-taking, further evaluations, and the composition of professional personnel were significantly different between the types of institutions. In addition, whether or not to perform adjuvant radiation therapy after surgery for rectal cancer was also significantly different according to the institution. These indicators were mainly available in large hospitals, but they were difficult to implement in small-sized hospitals because of inadequate and unskilled workforces. Subsequently, the inter-institutional variation was improved in most of the indicators for 6 years until the 2017 evaluation. In 2013, there was little improvement in the rates in general hospitals compared to other institutions leading to a large variation temporarily, but the trend gradually improved. At the national level, the quality of cancer treatment in each institution was graded, and a quality assessment was conducted to reduce the variation between institutions and improve the overall quality of patient treatments.
In addition, despite improvements in quality of indices, the variation in hospitalization costs and hospitalization days remained similar. This is likely to differ according to the severity or stage of the patient group at each type of institution. This is supported by the linear regression analysis results, in which the mortality rate showed a positive relationship between hospitalization cost and stay. In contrast, the surgical mortality rate was somewhat improved. Although this improvement in mortality has not been clearly seen in 6 years, it can be expected that such an improvement in the quality of treatment for each organ will result in an improvement in oncological outcomes.
The development of quality indicators for CRC has been carried out in a few western countries. Quality indicators have been developed in Europe and the United States since 2000s [9–12]. In Germany, 52 indicators were reported in 2013. The RAND/UCLA Appropriateness Method emphasized multidisciplinary composition and discussion, and attempted to connect ambulatory and hospital services that could be reflected taking into account the characteristics of European outpatient and medical care [12]. The Korean HIRA aims to reduce the variability of diagnostic and treatment quality, not health service accessibility, by institution, and the indicators are limited to the Korean medical environment. Therefore, there are some limitations to apply these to other western countries. It is necessary to present a revised quality assurance program that can improve not only quality of care but also health service accessibility through evidence-based guidelines worldwide.
Additional analyzes were performed on the process indicators that could affect surgical mortality and showed a negative correlation with the regional lymph node resection and examination rates. The Korean HIRA recommends evaluating a minimum of 12 lymph nodes to determine the exact nodal stage. The results of previous studies showed that a dissection of more than 12 lymph nodes was associated with an improved survival rate [13,14]. In our study, lymph node dissection also has been significantly associated with surgical mortality. There have been debates about the optimal number of resected lymph nodes in CRC. There were some studies showing that more lymph node dissection was needed in right hemicolectomy, and others showed that more lymph nodes should be removed if necessary depending on the presence of positive lymph nodes [15–17]. Proposing a resection of twelve lymph nodes according to the current guideline may be appropriate in evaluating the quality of treatment and reducing the variability by institution. However, additional clinical studies will be needed on the relevant number of resected lymph nodes according to the location of tumor with an improved survival in the future. There was no significant change in surgical mortality during the 6-year follow-up period, and a short follow-up period would limit the ability to analyze meaningful indicators [18,19]. However, in this study, we demonstrated the possibility of quality improvement by analyzing the indicators that were most correlated with surgical mortality and weighted them in the grading assessment.
In conclusion, the current treatment and examination status for CRC in South Korea have been improved, and this study is expected to be the basis for developing an relevant quality assessment program worldwide for CRC.
NotesEthical Statement Approval of an Institute Review Board was waived because the patient data were collected from administrative data without identifiable personal information. AcknowledgmentsThe authors wish to acknowledge the financial support of the St. Vincent’s Hospital, The Catholic University of Korea, Research institute of medical science foundation (SVHR-2019-08). This study is base on quality assessment research data of Korean Health Insurance Review & Assessment Service.
Fig. 3Trends in the coefficient of variation for each indicator. (A) Composition and pretreatment evaluation. Surgery-related (B), chemotherapy-related (C), radiotherapy-related (D), and outcome (E) indices. CEA, carcinoembryonic antigen. 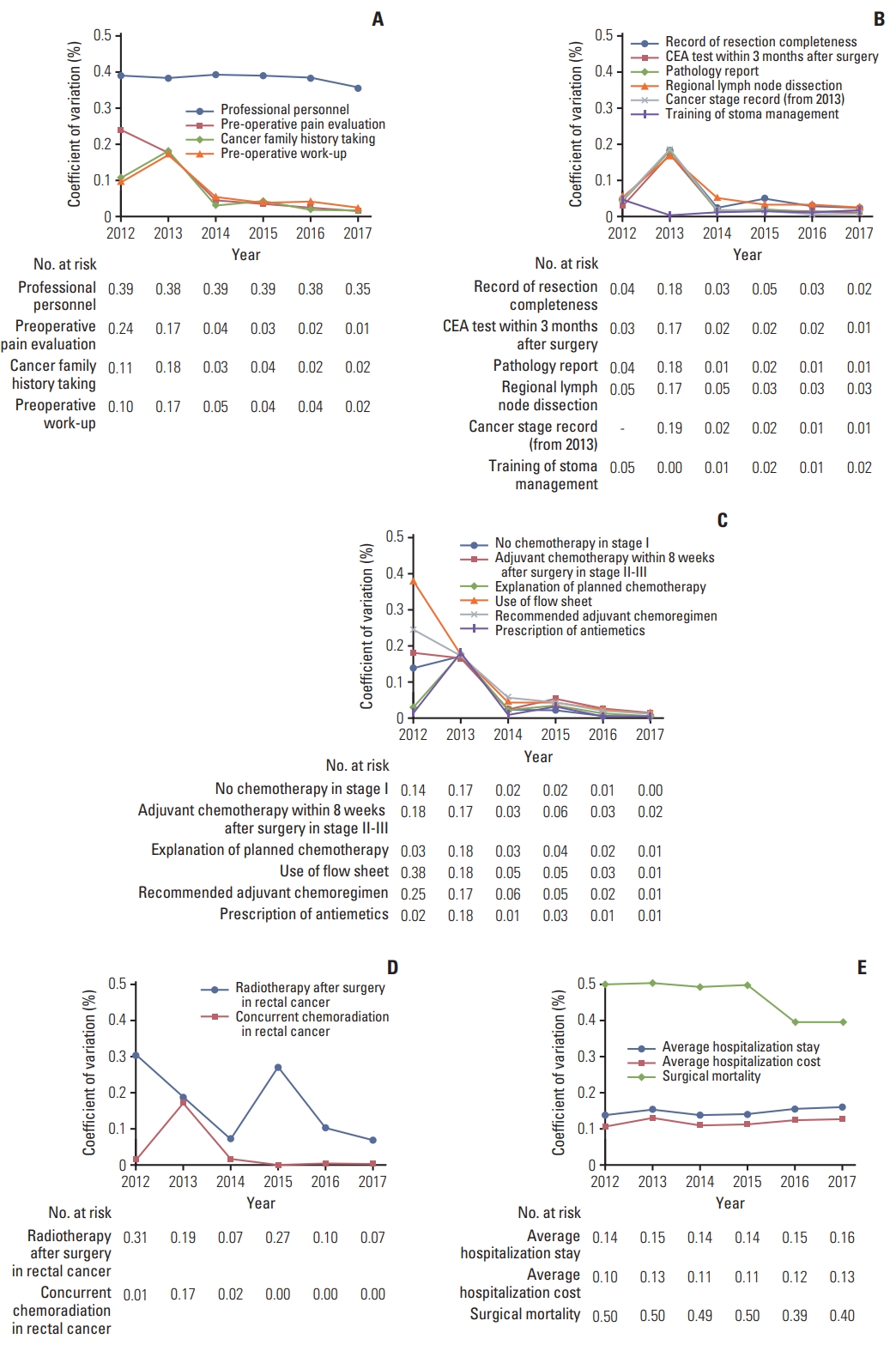
Fig. 4Graph of the linear regression analysis for surgical mortality. (A) Regional lymph node dissection. (B) Hospitalization stay. (C) Hospitalization cost. 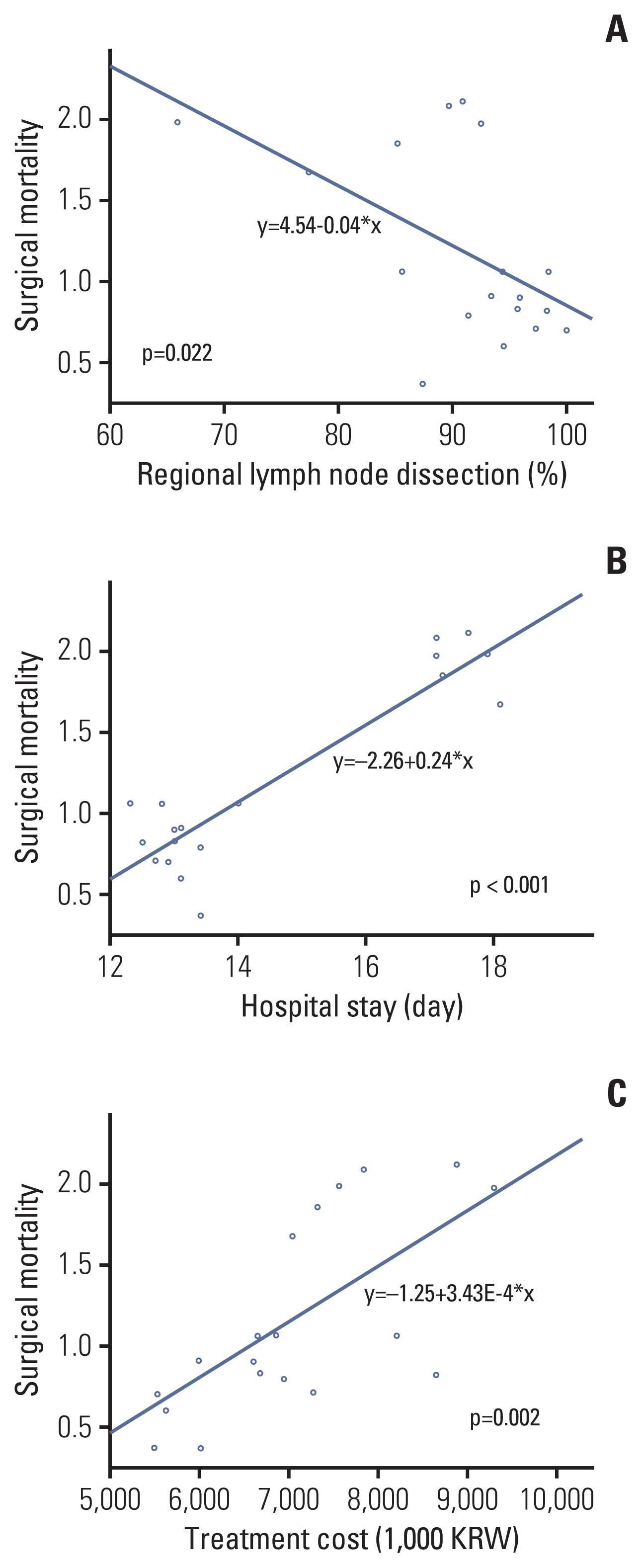
Table 1Study population
References1. Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020;52:335–50.
2. Keum N, Giovannucci E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. 2019;16:713–32.
3. Swedish Rectal Cancer Trial; Cedermark B, Dahlberg M, Glimelius B, Pahlman L, Rutqvist LE, et al. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980–7.
4. Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–46.
5. Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–40.
6. van de Velde CJ, Boelens PG, Borras JM, Coebergh JW, Cervantes A, Blomqvist L, et al. EURECCA colorectal: multidisciplinary management: European consensus conference colon and rectum. Eur J Cancer. 2014;50:1.
7. Banks I, Weller D, Ungan M, Selby P, Aapro M, Beishon M, et al. ECCO essential requirements for quality cancer care: primary care. Crit Rev Oncol Hematol. 2019;142:187–99.
8. De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, et al. Cancer survival in Europe 1999–2007 by country and age: results of EUROCARE-5: a population-based study. Lancet Oncol. 2014;15:23–34.
9. Malin JL, Asch SM, Kerr EA, McGlynn EA. Evaluating the quality of cancer care: development of cancer quality indicators for a global quality assessment tool. Cancer. 2000;88:701–7.
10. McGory ML, Shekelle PG, Ko CY. Development of quality indicators for patients undergoing colorectal cancer surgery. J Natl Cancer Inst. 2006;98:1623–33.
11. Dixon E, Armstrong C, Maddern G, Sutherland F, Hemming A, Wei A, et al. Development of quality indicators of care for patients undergoing hepatic resection for metastatic colorectal cancer using a Delphi process. J Surg Res. 2009;156:32–8.
12. Ludt S, Urban E, Eckardt J, Wache S, Broge B, Kaufmann-Kolle P, et al. Evaluating the quality of colorectal cancer care across the interface of healthcare sectors. PLoS One. 2013;8:e60947.
13. Compton CC. Key issues in reporting common cancer specimens: problems in pathologic staging of colon cancer. Arch Pathol Lab Med. 2006;130:318–24.
14. Washington MK, Berlin J, Branton P, Burgart LJ, Carter DK, Fitzgibbons PL, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009;133:1539–51.
15. Shen SS, Haupt BX, Ro JY, Zhu J, Bailey HR, Schwartz MR. Number of lymph nodes examined and associated clinicopathologic factors in colorectal carcinoma. Arch Pathol Lab Med. 2009;133:781–6.
16. Yang L, Xiong Z, Xie Q, He W, Liu S, Kong P, et al. Prognostic value of total number of lymph nodes retrieved differs between left-sided colon cancer and right-sided colon cancer in stage III patients with colon cancer. BMC Cancer. 2018;18:558.
17. Chen YJ, Yeh ST, Kao PS, Ou LH, Lin CS. A reappraisal of lymph node dissection in colorectal cancer during primary surgical resection. World J Surg Oncol. 2020;18:97.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||