AbstractPurposeThis study evaluated the re-challenge of S-1 or cisplatin in combination with docetaxel in metastatic gastric cancer (MGC) that had progressed on a cisplatin plus either S-1 or capecitabine regimen.
Materials and MethodsPatients with progressive disease after first-line cisplatin plus S-1 or capecitabine were randomized to receive 3-week cycles of docetaxel 75 mg/m2 intravenously (IV) on D1 (D), docetaxel 60 mg/m2 IV plus cisplatin 60 mg/m² IV on D1 (DC), or docetaxel 60 mg/m2 IV D1 plus oral S-1 30 mg/m2 twice a day on D1-14 (DS).
ResultsSeventy-two patients were randomized to the D (n=23), DC (n=24), or DS (n=25) group. The confirmed response rate was 4.3% (95% confidence interval [CI], 0% to 12.6%), 4.3% (95% CI, 0% to 12.6%), and 8.7% (95% CI, 0% to 20.2%) for the D, DC, and DS groups, respectively. Compared to the D arm, the DS arm had a better progression-free survival (2.7 months vs. 1.3 months, p=0.034) without any deterioration in safety or quality of life, whereas the DC arm had a similar progression-free survival (1.8 months vs. 1.3 months, p=0.804) and poorer overall survival (5.6 months vs. 10.0 months, p=0.035).
IntroductionDespite its decreasing incidence, gastric cancer remains a major global health issue with 951,600 new cases and 723,100 deaths in 2012 [1]. Although palliative chemotherapy has been established as the standard of care in patients with unresectable locally advanced or metastatic gastric cancer (MGC) based on the survival benefits and improved quality of life (QoL) over supportive care alone [2], the median overall survival (OS) of these patients rarely exceeds 12 months. Currently, fluoropyrimidine plus platinum-based combination chemotherapy is the most commonly used first-line reference regimens [3-6]. Recently, with the availability of oral fluoropyrimidines, such as capecitabine and S-1, infusional 5-fluorouracil (5-FU) has been substituted with either capecitabine or S-1, based on the results of phase III studies showing comparable efficacy and more favorable safety profiles with those agents [3-5,7]. The combination of capecitabine or S-1 plus cisplatin is currently one of the most commonly used first-line regimens in both clinical trials and practice. On the other hand, as the disease in most patients inevitably progresses during or after first-line chemotherapy, the role of salvage chemotherapy in gastric cancer has been investigated. Recent randomized phase III studies have revealed a significant survival benefit from second-line chemotherapy with docetaxel or irinotecan [8-10]. Nevertheless, given the modest survival benefits from current secondline chemotherapy, there is still a great need for further treatment improvements after the failure of first-line therapy.
Docetaxel, which inhibits microtubule depolymerization, has been used widely in the treatment of MGC. In particular, based on its different mechanism of action from those of fluoropyrimidine and platinum and the lack of cross-resistance with these agents, docetaxel is used frequently as a secondline regimen after the failure of fluoropyrimidine- and/or platinum-based first-line therapy. Furthermore, docetaxel shows synergistic antitumor activity with fluoropyrimidines, particularly S-1, by modulating the expression of enzymes involved in the 5-FU metabolism, including thymidylate synthase, dihydropyrimidine dehydrogenase, and orotate phosphoribosyltransferase [11,12]. Docetaxel has also shown synergistic activity with cisplatin in gastric cancer, which was attributed partially to the suppression of the cisplatin-induced multidrug resistance-associated protein-1 by docetaxel [13], resulting in the accumulation of intracellular platinum-glutathione complexes. Given that these molecules modulated by docetaxel are involved in the resistance to fluoropyrimidines and cisplatin in gastric cancer [14,15], the hypothesis in this study was that the co-treatment of docetaxel and either S-1 or cisplatin could increase the antitumor activity compared to docetaxel alone, by at least partially overcoming the resistance to fluoropyrimidines or cisplatin in patients whose tumors progressed during or after fluoropyrimidines- or cisplatin-based first-line therapy.
Materials and Methods1. PatientsEligible patients were ≥ 18 years old with histologically confirmed metastatic gastric adenocarcinoma. The patients needed to have documented disease progression during or within 6 months of the completion of first-line chemotherapy with either S-1 or capecitabine plus cisplatin. The additional eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, a measurable lesion, adequate major organ function, absence of concurrent uncontrolled medical conditions or other malignancies within the past 3 years, prior taxane treatment, and preexisting grade ≥ 2 neuropathy. All patients provided written informed consent prior to study entry. The institutional review boards of all participating centers approved the study protocol (ClinicalTrials.gov identifier NCT00980603).
2. Study design and treatmentThe patients were assigned randomly 1:1:1 to receive docetaxel (75 mg/m2 intravenously on day 1), docetaxel plus cisplatin (each 60 mg/m2 intravenously on day 1), or docetaxel plus S-1 (docetaxel 60 mg/m2 intravenously on day 1 and oral S-1 30 mg/m2 twice daily on days 1-14), administered every 3 weeks. The dose of docetaxel in the docetaxel alone arm was based on a previous phase II study of MGC in a second-line setting [16]. The doses of triweekly docetaxel plus cisplatin in previous studies were 60-75 mg/m2 and 60-70 mg/m2, respectively, in a second-line setting [17-20]. Based on the safety and efficacy of these trials, the dose in the docetaxel plus cisplatin arm was determined to be 60 mg/m2 each for the current trial. In the docetaxel plus S-1 arm, although the recommended doses of triweekly S-1 plus docetaxel were S-1 80 mg/m2/day on days 1 to 14 and docetaxel 40 mg/m2 on day 1 in previous studies [21,22], this study used the same dose of docetaxel (60 mg/m2) as in the docetaxel plus cisplatin arm, along with a reduced dose of S-1 (60 mg/m2/day).
Randomization was performed using the random permutation method to stratify the patients according to the study site, ECOG performance status (0-1 vs. 2), and the best response to first-line chemotherapy (complete or partial response vs. stable disease or progressive disease). Treatment was continued until disease progression, unacceptable toxicity, or the withdrawal of consent.
3. AssessmentsThe medical history, physical examination, and laboratory tests (including a complete blood count with differential, chemistry [including creatinine clearance], and urinalysis) were performed within 1 week before the study treatment. The physical examinations, and the blood hematology and chemistry tests were repeated at the beginning of each cycle. The baseline tumor assessment using the chest X-ray and abdominal/pelvic computed tomography scans were performed within 4 weeks before the study treatment, and these imaging methods were repeated every two treatment cycles until disease progression. The objective tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.0 [23], and all responses were confirmed at least 4 weeks after the initial documentation.
The safety evaluations were based on the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) ver. 3.0. The QoL was assessed using the European Organization for Research and Treatment of Cancer QoL questionnaires (EORTC QLQ-C30 and gastric module STO22) at the beginning of each cycle for the initial four cycles, and then every other cycle until disease progression.
4. Statistical analysisThe primary endpoint of this study was the objective response rate (ORR). The secondary endpoints included the progression-free survival (PFS; time from the date of start treatment to the date of disease progression or death), OS (time from the date of start treatment to the date of death), safety, and QoL. Based on Simon’s selection design with a probability of 90% for correctly selecting the best treatment with respect to ORR, assuming that the smallest ORR would be 16% and the best treatment would be superior by an absolute difference of 14% (i.e., an ORR of 30%), a total of 144 patients including a 5% dropout rate (i.e., 48 patients per treatment group) were found to be required.
Patients who received at least one dose of the study drug comprised the safety population. Efficacy analysis was performed in a modified intention-to-treat (ITT) population, which excluded patients who were deemed ineligible or never started the study treatment from randomized patients.
The Kaplan-Meier method and the log-rank test were used to estimate and compare the survival distribution, respectively. The discrete data were compared using a Pearson’s chi-square test or Fisher exact test, and the quantitative data were compared using the Kruskal-Wallis test. All tests were two-sided with a 5% significance level.
Results1. Patient characteristicsSeventy-two patients (50% of the target number) from three institutions were enrolled between November 2008 and September 2012. The restriction of accrual to a previous failed regimen of cisplatin plus either S-1 or capecitabine resulted in slow accrual, which caused early closure of the study. The patients were assigned randomly to the docetaxel alone (D; n=23), docetaxel plus cisplatin (DC; n=24), or docetaxel plus S-1 (DS; n=25) groups. One patient in the DC arm was deemed ineligible because of prior first-line chemotherapy with capecitabine plus oxaliplatin rather than cisplatin. This patient was excluded from the efficacy analysis but included in the safety analysis. Two patients in the DS arm were also excluded from the efficacy and toxicity analyses because of a refusal to receive chemotherapy after randomization in one case and the receipt of a second-line chemotherapy with infusional 5-FU instead of S-1 in the other patient (Fig. 1). The baseline characteristics were well balanced between the treatment arms (Table 1). The majority of patients initially had metastatic disease with multiple metastatic organ sites and had developed disease progression within 6 months during their first-line chemotherapy.
2. Treatment deliveryThe median number of treatment cycles was 2 (range, 1 to 10) in the D arm, 2 (range, 1 to 11) in the DC arm, and 3 (range, 1 to 23) in the DS arm. The proportion of patients requiring a dose reduction was higher in the DC and DS arms than in the D arm: 21.7% (5 of 23) with D, 30.4% (7 out of 23) with DC, and 34.8% (8 of 23) with DS. The most common cause of dose reduction was neutropenia (2 of 5) in the D arm, neutropenia (2 of 7) in the DC arm, and mucositis (3 of 8) in the DS arm. The proportion of patients who experienced cycle delay owing to adverse events was similar in the three arms: 26.1% (6 of 23) with D, 21.7% (5 of 23) with DC, and 26.1% (6 of 23) with DS. The median relative dose intensities were 92.3% for docetaxel in the D arm, 90.6% for docetaxel and 90.6% for cisplatin in the DC arm, and 91.2% for docetaxel and 89.1% for S-1 in the DS arm. The main reasons for the discontinuation of treatment were disease progression (95.7% [n=22] with D, 100% [n=23] with DC, and 91.3% [n=21] with DS) followed by adverse events (4.3% [n=1] with D, 0% with DC, and 4.3% [n=1] with DS).
3. EfficacyAmong the modified ITT population, confirmed ORR was 4.3% (1 of 23; 95% confidence interval [CI], 0% to 12.6%) in the D arm, 4.3% (1 of 23; 95% CI, 0% to 12.6%) in the DC arm, and 8.7% (2 of 23; 95% CI, 0% to 20.2%) in the DS arm (p > 0.990) (Table 2). The ORR, including the unconfirmed partial response, was 4.3% (1 of 23; 95% CI, 0% to 24.4%) in the D arm, 13.0% (3 of 23; 95% CI, 0% to 32.9%) in the DC arm, and 13.0% (3 of 23; 95% CI, 0% to 32.9%) in the DS arm (p=0.685). The disease control rate (DCR; the percentage of patients who achieved a complete response, partial response and stable disease) was 30.4% (7 of 23; 95% CI, 11.6% to 49.2%) in the D arm, 56.5% (13 of 23; 95% CI, 36.2% to 76.8%) in the DC arm, and 52.2% (12 of 23; 95% CI, 31.8% to 72.6%) in the DS arm (p=0.164). With a median follow-up time of 7.3 months (range, 1.6 to 31.5), the median PFS was 1.3 months with D (95% CI, 1.0 to 1.5), 1.8 months with DC (95% CI, 0.8 to 2.9), and 2.7 months with DS (95% CI, 1.0 to 4.4; p=0.072) (Fig. 2A). The DS arm showed a significantly prolonged PFS compared to the D arm (p=0.034), whereas the DC arm did not show a significant difference in PFS compared to the D arm (p=0.804). In terms of OS, the DC arm was worse than the D arm (median, 5.6 months [95% CI, 4.4 to 6.7] vs. 10.0 months [95% CI, 7.8 to 12.2]; p=0.035), whereas the DS arm (median, 6.9 months; 95% CI, 2.1 to 11.7) was comparable to the D arm (p=0.421) (Fig. 2B).
4. Post-study subsequent therapyThe proportion of patients receiving third-line therapy was higher in the D arm (19 of 23, 82.6%) than in the DC (12 of 23, 52.2%) or DS arms (13 of 23, 56.5%; p=0.089). The ORR in the third-line therapy was 10.5% (2 of 19; 95% CI, 0% to 24.3%) for D, 0.0% (0 of 13) for DC, and 0.0% (0 of 13) for DS (p=0.637).
5. Safety
Table 3 lists the adverse events. The overall incidence of grade ≥ 3 events was 43.5% (10 of 23) in the D arm and 62.5% (15 of 24) in the DC arm, and 32.9% (8 of 23) in the DS arm (DC vs. D, p=0.155; DS vs. D, p=0.382). Although the overall toxicity profiles in the treatment arms were comparable, the incidence of all grade nausea was significantly higher in the DS arm than the D arm (44% vs. 13%, p=0.047). The incidence of all grade aminotransferases elevation (42% vs. 13%, p=0.049) was significantly higher in the DC arm, whereas infection without neutropenia occurred more frequently in the D arm than in the DC arm (26% vs. 4%, p=0.048).
The most common grade 3/4 toxicity was neutropenia (21.7% with D, 25.0% with DC, and 8.7% with DS); febrile neutropenia occurred in 8.7% of cases in the D arm, 8.3% in the DC arm, and 4.3% in the DS arm. There was one possible treatment-related death in each of the DC (infection without neutropenia, peritonitis) and DS arms (infection with neutropenia, pneumonia).
6. Quality of lifeMore than 60% of the patients in each arm completed the baseline QoL questionnaire and at least one post-treatment questionnaire. QoL compliance was similar in the three arms. Although there were no significant differences in the global QoL scores between the three arms, the combination arms had poorer QoL scores at some time points compared to the D arm. These included lower physical functioning at cycle 2 (p=0.002), role functioning at cycles 2 (p=0.014) and 3 (p=0.018), and emotional functioning at cycle 3 (p=0.014), more fatigue at cycles 2 (p=0.010) and 3 (p=0.049), appetite loss at cycle 2 (p=0.005), insomnia at cycle 3 (p=0.021), and dry mouth at cycle 2 (p=0.028) in the DC arm. Fatigue at cycles 2 (p=0.040) and 3 (p=0.018), constipation at cycle 2 (p=0.047), nausea/vomiting at cycle 3 (p=0.045), reflux symptoms at cycle 2 (p=0.009), and hair loss at cycle 3 (p=0.017) were elevated in the DS arm (Fig. 3).
DiscussionThe reported survival benefits of second-line chemotherapy in patients with MGC have prompted efforts to develop more effective second-line chemotherapy regimens. Recent phase III studies demonstrating survival improvement with second- or third-line therapy compared to the best supportive care used monotherapies, such as irinotecan or docetaxel alone [8-10]. In the previous COUGAR-02 phase III trial, docetaxel also had health-related QoL benefits [9]. On the other hand, there is no single standard second-line regimen because survival was not found to differ between irinotecan and docetaxel or between irinotecan and paclitaxel in a salvage setting [10,24]. Based on the survival gain from secondline single agents being modest and S-1 plus cisplatin, resulting in better survival than S-1 alone in a first-line setting [6,8-10], combination regimens have also been evaluated in a second-line setting. In a phase III study (TCOG GI-0801/BIRIP) that compared irinotecan alone with irinotecan plus cisplatin in patients with metastatic or recurrent gastric cancer refractory to S-1–based first-line chemotherapy, the PFS (3.8 months vs. 2.8 months; hazard ratio [HR], 0.68; p=0.0398) and the DCR (75% vs. 54%, p=0.0162) were significantly better in the irinotecan plus cisplatin group than in the irinotecan alone group, whereas there was no difference in survival between the two groups [25]. In another phase III trial (TRICS), which also compared irinotecan alone with irinotecan plus cisplatin in advanced gastric cancer refractory to first-line S-1 monotherapy, the addition of cisplatin to irinotecan did not improve the PFS (4.6 months vs. 4.1 months; HR, 0.860; p=0.376) or OS (13.9 months vs. 12.7 months; HR, 0.834; p=0.288) compared to irinotecan monotherapy [26].
Based on the preclinical synergism between docetaxel and either S-1 or cisplatin [11-15], it was hypothesized that a combination of docetaxel and either S-1 or cisplatin as a secondline treatment might have better efficacy than docetaxel alone, even after the failure of first-line cisplatin plus either S-1 or capecitabine. On the other hand, compared to docetaxel alone, the addition of cisplatin to docetaxel did not show any improvement in the ORR (4.3% in the DC arm vs. 4.3% in the D arm; p > 0.990), PFS (median, 1.8 months vs. 1.3 months; p=0.804), or OS (median, 5.6 months vs. 10.0 months; p=0.035), whereas this combination led to a deterioration in some QoL scores, including physical functioning, role functioning, emotional functioning, fatigue, appetite loss, insomnia, and dry mouth.
In contrast, the addition of S-1 to docetaxel showed a better PFS (median, 2.7 months vs. 1.3 months; p=0.034) than docetaxel alone without substantial impairment in the QoL or increasing toxicity except for all grades of nausea. Although this PFS benefit was not translated to an OS benefit, this effect might have been due to the higher proportion of patients who received subsequent chemotherapy in the D arm than in the DS arm (82.6% vs. 56.5%). In terms of confirmed ORR, which is similar to the addition of cisplatin to docetaxel, the addition of S-1 to docetaxel showed a very low ORR and did not show any significant difference compared to docetaxel alone (4.3% in the D arm, 4.3% in the DC arm, and 8.7% in the DS arm; p > 0.990). Previous studies of second-line chemotherapy in MGC showed an ORR ranging from 0% to 22% [8,9,24-27], and the ORR of the present study was much lower than the ORRs of previous studies. The small sample size might have affected the result because the baseline characteristics and treatment delivery did not appear to differ from previous studies. On the other hand, after including the unconfirmed response, the ORRs of the combination arms appeared to be comparable to those of previous studies (4.3% in the D arm vs. 13.0% in the DC arm vs. 13.0% in the DS arm; p=0.685).
A recent phase III study (JACCRO GC-05) also evaluated the re-introduction of a previous failed drug (S-1) combined with a new agent (irinotecan) in advanced gastric cancer refractory to first-line S-1 treatment [27]. The combination of irinotecan with S-1 did not show any PFS (3.8 months vs. 3.4 months; HR, 0.85; p=0.16) or OS (8.8 months vs. 9.5 months; HR, 0.99; p=0.92) benefits compared to irinotecan alone, even though grade ≥ 3 leukopenia and febrile neutropenia were significantly higher with the combination regimen. These inconsistent results regarding the re-introduction of a failed drug were also reported in earlier studies of metastatic colorectal cancer. In one report, the combination of oxaliplatin with infusional 5-FU/leucovorin resulted in a significantly better ORR (9.9% vs. 1.3% vs. 0%) and time to progression (median, 4.6 months vs. 1.6 months vs. 2.7 months) than oxaliplatin or infusional 5-FU/leucovorin alone following progression on irinotecan plus bolus 5-FU/leucovorin [28]. In another report, however, the combination of irinotecan with infusional 5-FU/leucovorin did not lead to an improvement in the ORR (16% vs. 11%, p=0.07), PFS (median, 4.4 months vs. 4.3 months; p=0.75), or OS (median, 15.0 months vs. 13.9 months; p=0.16) compared to irinotecan alone after the failure of first-line infusional 5-FU/leucovorin [29]. Currently, in colorectal cancer treatment regimens, 5-FU/leucovorin is normally re-administered after a previous failure in combination with either irinotecan or oxaliplatin [30].
The present study had several limitations. This was a small sized phase II study with an unmet primary endpoint and was terminated early because of the slow accrual. With these limitations, conclusions could not be drawn regarding the role of adding a previous failed agent to a second-line therapy in MGC. Given its importance in clinical practice, this issue needs to be addressed further in future clinical trials.
ConclusionThe addition of S-1, but not cisplatin, to docetaxel as a second-line treatment resulted in better efficacy in terms of the PFS compared to docetaxel alone, without clinically significant impairment of safety or QoL aspects, in MGC patients after progression on first-line S-1 or capecitabine plus cisplatin. Given that the re-challenge issue beyond progression might be dependent on a specific agent, molecularly targeted agents as well as cytotoxic chemotherapy will need to be investigated further in this setting to better optimize the second-line regimens in gastric cancer.
AcknowledgmentsThis study was supported by the Research Institute and Hospital, National Cancer Center, Republic of Korea (grant 1010180).
Fig. 1.CONSORT diagram. D, docetaxel; DC, docetaxel plus cisplatin; DS, docetaxel plus S-1; 5-FU, 5-fluorouracil. a)One ineligible patient who had received capecitabine plus oxaliplatin as first-line therapy was given the allocated study treatment (DC) and was included in the safety analysis. 
Fig. 2.Kaplan-Meier curves of progression-free survival (A) and overall survival (B). CI, confidence interval; DS, docetaxel plus S-1; DC, docetaxel plus cisplatin; D, docetaxel. 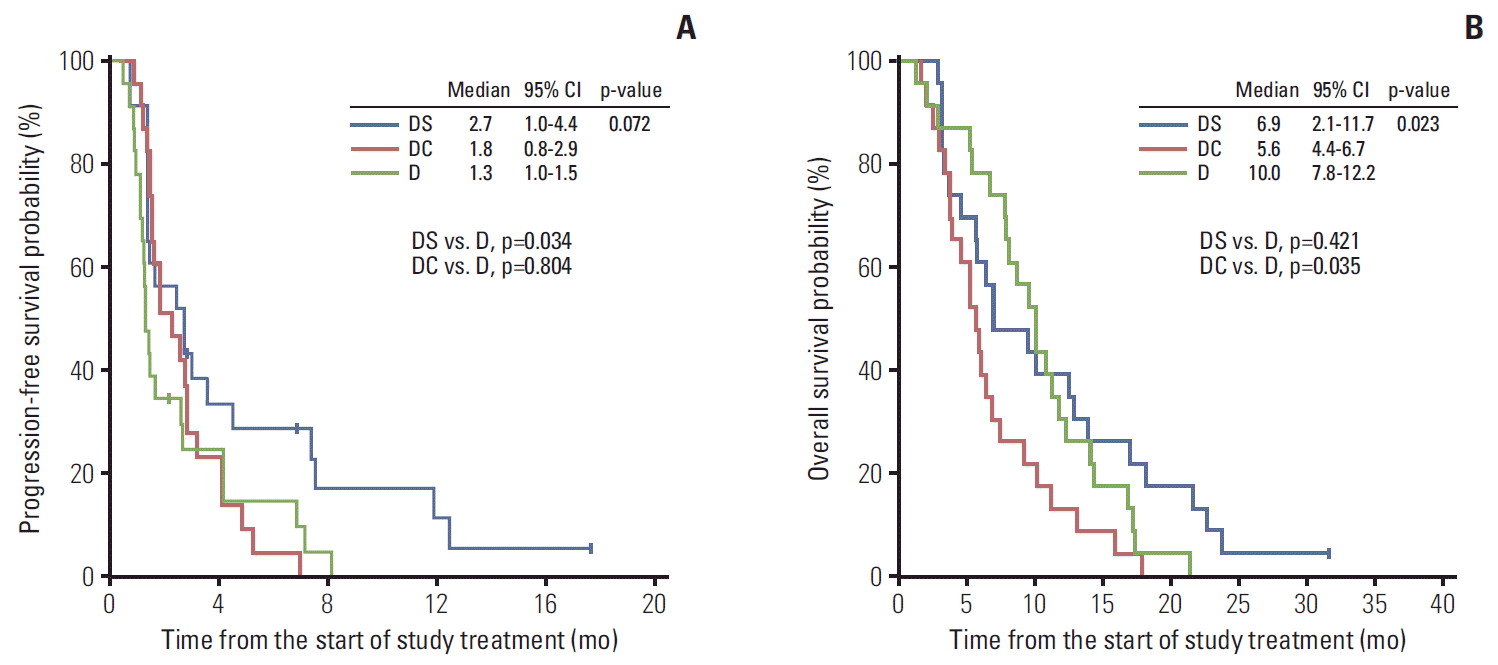
Fig. 3.Mean quality of life scores by the treatment arms. Global quality of life (A), physical functioning (B), role functioning (C), emotional functioning (D), nausea and vomiting (E), fatigue (F), appetite loss (G), and reflux symptoms (H). For the global quality of life, physical, role, or emotional functioning, higher scores indicate better quality of life or functioning. For nausea and vomiting, fatigue, appetite loss, and reflux symptoms, higher scores indicate a higher level of symptoms. D, docetaxel; DC, docetaxel plus cisplatin; DS, docetaxel plus S-1. *p < 0.05. 
Table 1.Baseline characteristics Table 2.Objective tumor response
Table 3.Adverse events
References1. Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–41.
2. Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, et al. Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8:163–8.
3. Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as firstline therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–73.
4. Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46.
5. Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–53.
6. Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–21.
7. Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10:1063–9.
8. Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer: a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011;47:2306–14.
9. Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86.
10. Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–8.
11. Takahashi I, Emi Y, Kakeji Y, Uchida J, Fukushima M, Maehara Y. Increased antitumor activity in combined treatment TS-1 and docetaxel: a preclinical study using gastric cancer xenografts. Oncology. 2005;68:130–7.
12. Wada Y, Yoshida K, Suzuki T, Mizuiri H, Konishi K, Ukon K, et al. Synergistic effects of docetaxel and S-1 by modulating the expression of metabolic enzymes of 5-fluorouracil in human gastric cancer cell lines. Int J Cancer. 2006;119:783–91.
13. Maeda S, Sugiura T, Saikawa Y, Kubota T, Otani Y, Kumai K, et al. Docetaxel enhances the cytotoxicity of cisplatin to gastric cancer cells by modification of intracellular platinum metabolism. Cancer Sci. 2004;95:679–84.
14. Takiuchi H, Kawabe S, Gotoh M, Katsu K. Thymidylate synthase gene expression in primary tumors predicts activity of s-1-based chemotherapy for advanced gastric cancer. Gastrointest Cancer Res. 2007;1:171–6.
15. Endo K, Maehara Y, Kusumoto T, Ichiyoshi Y, Kuwano M, Sugimachi K. Expression of multidrug-resistance-associated protein (MRP) and chemosensitivity in human gastric cancer. Int J Cancer. 1996;68:372–7.
16. Lee JL, Ryu MH, Chang HM, Kim TW, Yook JH, Oh ST, et al. A phase II study of docetaxel as salvage chemotherapy in advanced gastric cancer after failure of fluoropyrimidine and platinum combination chemotherapy. Cancer Chemother Pharmacol. 2008;61:631–7.
17. Kunisaki C, Imada T, Yamada R, Hatori S, Ono H, Otsuka Y, et al. Phase II study of docetaxel plus cisplatin as a second-line combined therapy in patients with advanced gastric carcinoma. Anticancer Res. 2005;25:2973–7.
18. Polyzos A, Tsavaris N, Kosmas C, Polyzos K, Giannopoulos A, Felekouras E, et al. Subsets of patients with advanced gastric cancer responding to second-line chemotherapy with docetaxel-cisplatin. Anticancer Res. 2006;26:3749–53.
19. Park SH, Kang WK, Lee HR, Park J, Lee KE, Lee SH, et al. Docetaxel plus cisplatin as second-line therapy in metastatic or recurrent advanced gastric cancer progressing on 5-fluorouracil-based regimen. Am J Clin Oncol. 2004;27:477–80.
20. Kim H, Park JH, Bang SJ, Kim DH, Cho HR, Kim GY, et al. A phase II study of docetaxel and cisplatin in patients with gastric cancer recurring after or progressing during 5-FU/platinum treatment. Jpn J Clin Oncol. 2005;35:727–32.
21. Yoshida K, Ninomiya M, Takakura N, Hirabayashi N, Takiyama W, Sato Y, et al. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res. 2006;12(11 Pt 1):3402–7.
22. Koizumi W, Kim YH, Fujii M, Kim HK, Imamura H, Lee KH, et al. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: a randomized study (START). J Cancer Res Clin Oncol. 2014;140:319–28.
23. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16.
24. Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438–44.
25. Higuchi K, Tanabe S, Shimada K, Hosaka H, Sasaki E, Nakayama N, et al. Biweekly irinotecan plus cisplatin versus irinotecan alone as second-line treatment for advanced gastric cancer: a randomised phase III trial (TCOG GI-0801/BIRIP trial). Eur J Cancer. 2014;50:1437–45.
26. Nishikawa K, Fujitani K, Inagaki H, Akamaru Y, Tokunaga S, Takagi M, et al. Randomised phase III trial of second-line irinotecan plus cisplatin versus irinotecan alone in patients with advanced gastric cancer refractory to S-1 monotherapy: TRICS trial. Eur J Cancer. 2015;51:808–16.
27. Tanabe K, Fujii M, Nishikawa K, Kunisaki C, Tsuji A, Matsuhashi N, et al. Phase II/III study of second-line chemotherapy comparing irinotecan-alone with S-1 plus irinotecan in advanced gastric cancer refractory to first-line treatment with S-1 (JACCRO GC-05). Ann Oncol. 2015;26:1916–22.
28. Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059–69.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||