INTRODUCTION
Adenoid cystic carcinoma (ACC) is a variant of adenocarcinoma that show distinct histopathologic and clinical features. It is thought to be the proliferation of myoepithelial and secretory epithelial cells, but its histogenesis has not been fully established (1). ACC most commonly occurs in the salivary glands and less commonly in other sites such as the breast, skin, uterine cervix, upper aero-digestive tract and lung (2,3). All of those ACCs show histological similarities regardless of their primary sites (1,3).
Primary ACCs in the lung are very rare, and this accounts for only 0.09~0.2% of all lung cancers (4). There are only a few reports about pulmonary ACCs and moreover, only a few of the reports has included the clinical courses of the patients who initially had distant metastases. Because of its rarity, the clinicopathologic characteristics of this disease usually have been presumed to be similar to those of salivary disease.
I would report here on the disease course and treatment outcome of a patient who was initially diagnosed as having primary pulmonary adenoid cystic carcinoma with liver metastases. The tumor showed extreme radiosensitivity and a very aggressive clinical course.
CASE REPORT
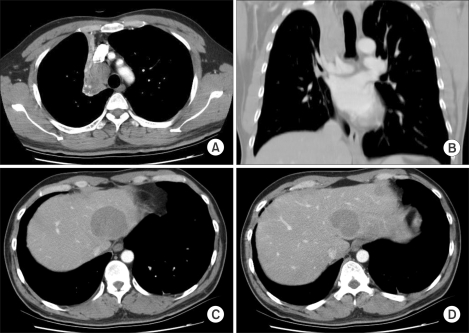
A 41-year-old man visited to a hospital late in December 2003 due to dull chest pain and minor hemoptysis that had lasted for the previous 2 months. On the chest CT scan, a lung mass that measured more than 4 cm was detected at the right upper lobe (RUL) and it involved the proximal main bronchus. The mass had conglomerated with the right paratracheal lymph nodes and it had caused RUL atelectasis. Simultaneously, three metastatic nodules were detected on both hepatic lobes (Fig. 1). On the 18F-FDG whole body PET scan, the FDG uptake was abnormally increased at all of those sites. Adenoid cystic carcinomas were identified in both lungs and as a liver mass, according to cytologic analysis with performing percutaneous needle aspiration (PCNA).
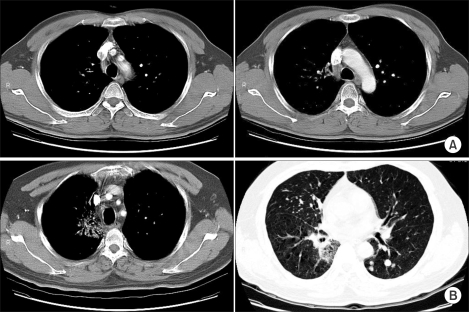
From February 16th, 2004, oral cyclophosphamide (250 mg bid) was started with the patient's consent. He was then referred to our department for palliative radiotherapy. From February 23 to April 14, 2004, a total dose of 66 Gy was delivered using 2 Gy fractions to the lung mass and to the enlarged mediastinal nodes. During thoracic radiotherapy, the cyclophosphamide was stopped after 1 week's use and the response of both the lung and liver masses was negligible. Those lesions were remarkably decreased during radiotherapy and at the end of treatment, they had nearly disappeared (Fig. 2A).
Bone metastases developed in this patient at 4 months from initial diagnosis. Several chemotherapeutic agents including 7 cycles of weekly paclitaxel (50 mg/m2, 90 mg) combined with CDDP (18 mg/m2, 30 mg) and 2 cycles of weekly docetaxel (50 mg/m2, 80 mg & 40 mg) were tried sequentially after detecting the bony metastases. Those agents showed responses better than minimal to the liver metastases. Yet the bone metastases progressed rapidly during chemotherapy. Multiple lung metastases were also detected at 12 month from the initial diagnosis (Fig. 2B). After that, Geftinib (250 mg daily) was tried, but there was no response. At 15 month, the cytologic analysis of the pleural fluid revealed malignant pleural effusion.
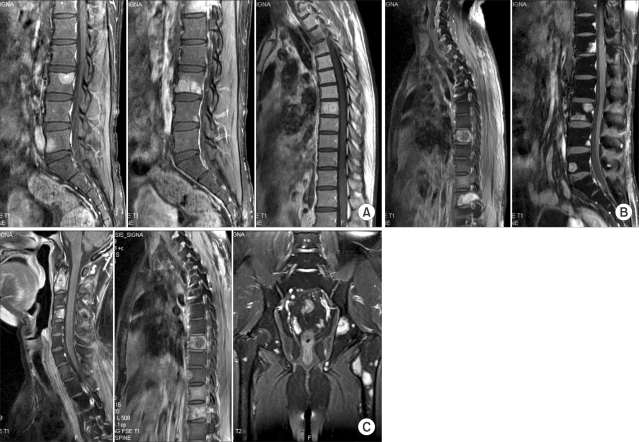
The bone metastases progressed very quickly and disseminated to the nearly the whole spine (except for the previously irradiated upper thoracic spine), the pelvis, femur and sternum at about 8.5 months in spite of continuing the chemotherapy (Fig. 3). For the painful sites, palliative radiotherapy was given with the doses that ranged from 25 to 35 Gy and with using 2.5 or 3.0 Gy fractions. The responses to radiotherapy were remarkably fast and good. Generally, the pain relief started after two or more fractions of radiotherapy and the symptoms at the end of treatment, including the pain, were almost completely resolved. The palliation of symptoms was sustained until death.
He died because of disease progression in the lung and the associated pneumonia at 20 months from the initial diagnosis. There was no evidence of loco-regional progression in the irradiated thorax until death.
DISCUSSION
Extra-salivary adenoid cystic carcinomas (ACCs) are very rare tumors and they have histological findings that are similar to ACC salivary disease. Therefore, their clinicopathological characteristics usually have been extrapolated from the salivary ACCs, regardless of the primary sites. However, Shin et al. reported that breast ACCs followed a favorable clinical course with a better prognosis than salivary disease (5), and Cerar et al. reported that esophageal ACCs had a worse prognosis because of the synchronous tumors of the esophagus and their vital location (6). The clinical behaviors of pulmonary ACCs have not been fully defined.
The optimal management for this unusual disease is surgical resection whenever feasible. The prognosis was generally poor with overall survival of 10~20% at 2 years in the past. However, with the improvement of surgical techniques, the 5 year survival rates of the resected cases have been reported to be 60~100%. But many patients are not surgical candidates, and if the tumor is resected, then some patients are at a high risk of local recurrence due to incomplete resection. Unlikely other lung cancers, some patients with pulmonary ACCs have prolonged survival despite of residual tumor, and most ACCs are radiosensitive (7).
Although it was originally classified as a bronchial adenoma, pulmonary ACC is actually a malignant tumor that may metastasize. Metastasis to the regional lymph nodes may be present in 10~20% of the newly diagnosed pulmonary ACCs (8). Distant metastasis usually occurs late in the disease course, and this is most commonly observed in the lung, bone, liver and brain (9).
According to a review of the literature, pulmonary ACC will not be necessarily indolent and it may occasionally follow a much more aggressive course than that generally ascribed to their salivary counterparts or to the metastatic pulmonary ACCs (2). Therefore, more aggressive approaches including radiotherapy and/or chemotherapy might be considered to be added much earlier at the initial time of treatment. Because of this heterogeneity of the prognosis, it would be more important that significant prognostic factors should be identified.
There has been controversy about the prognostic value of the histologic grade for the cases of salivary ACC and the other extra-salivary ACCs (2,10~13). This controversy also exists for the cases of pulmonary ACCs. Normori et al. reported that the histologic grade correlated with the growth pattern and the prognosis, and grade III tumors (>20% solid component) predominantly showed extra-luminal growth and distant failure (1). Albers et al. also showed the possible correlation between the histologic grade and treatment results (10). Yet Moran et al. reported that the disease stage at diagnosis, but not the histologic features (e.g., the pattern of growth, cytologic atypia and mitotic activity), predicted the clinical outcome (2). For lymph nodal metastases, there is no conclusive result, but some authors have reported no correlation between the lymph node status and survival (8,9). There has been no report about the prognostic significance for the specific site of distant metastases. For the cases of head and neck ACCs, the presence of bone metastasis was reported as a possible poor prognostic factor and the median survival after bone metastases was only 21 months (54.0 months for lung metastases) (12) van der Wal et al. also reported that extrapulmonary metastasis (e.g. bones and brains) was an adverse prognostic factor for salivary ACCs and the median survival after bone metastases was 13.5 months (25.0 months for lung metastases) (14). For this report's patient, the initial distant metastases and also the nodal metastases might be considered as possible poor prognostic factors in light of the previously published reports. As for the specific sites of metastases as a prognostic factor, the bone metastases were more refractory to chemotherapy than the other metastatic sites and the bone metastases progressed very quickly. The patient survived 16 months after the diagnosis of bone metastases. Considering these findings, bone metastases might be considered as a possible poor prognostic factor for pulmonary ACCs.
Some markers have been evaluated as possible prognostic factors. Ki-67 is a marker for cell proliferation and it has been used as an adjunct to the histologic grade or as an indicator of progression for some tumors. However, there were no significant correlations among the histologic grade, the Ki-67 score or CD 117 positivity and the overall survival according to Albers' report (10). Lin et al. reported that DNA ploidy and the S phase fractions might be correlated with the tumor grade and metastasis, and there was no overexpression of Her-2/neu, p53 and COX-2 in tracheobronchial ACCs (15).
Despite of the need to find prognostic factors, all of previously reported results can not be conclusive because most of the studies had a very small numbers of patients over a long time period. So, large scaled multi-institutional trials are needed to investigate the prognostic factors of pulmonary ACCs.
Radiotherapy was an effective modality for this study's patient, who had pulmonary adenoid cystic carcinoma with distant metastases. Adenoid cystic carcinoma is generally known to be a slowly growing tumor, but it may disseminate very rapidly. Therefore, the factors predicting aggressive behavior should be determined and treatment might be individualized according to the primary sites and on a patient-by-patient basis.