AbstractEvidence suggests that combined gemcitabine-cisplatin chemotherapy extends survival in patients with advanced biliary tract cancer (BTC). We conducted a systematic review in order to collate this evidence and assess whether gemcitabine-cisplatin efficacy is influenced by primary tumor site, disease stage, or geographic region, and whether associated toxicities are related to regimen. MEDLINE (1946-search date), EMBASE (1966-search date), ClinicalTrials. gov (2008-search date), and abstracts from major oncology conferences (2009- search date) were searched (5 Dec 2013) using terms for BTC, gemcitabine, and cisplatin. All study types reporting efficacy (survival, response rates) or safety (toxicities) outcomes of gemcitabine-cisplatin in BTC were eligible for inclusion; efficacy data were extracted from prospective studies only. Evidence retrieved from one meta-analysis (abstract), four randomized controlled trials, 12 nonrandomized prospective studies, and three retrospective studies supported the efficacy and safety of gemcitabine-cisplatin for BTC. Median overall survival ranged from 4.6 to 11.7 months, and response rate ranged from 17.1% to 36.6%. Toxicities were generally acceptable and manageable. Heterogeneity in study designs and data collected prevented formal meta-analysis, however exploratory assessments suggested that efficacy did not vary with primary tumor site (gallbladder vs. others), disease stage (metastatic vs. locally advanced), or geographic origin (Asia vs. other). Incidence of grade 3/4 toxicities was not related to gemcitabine dose or cisplatin frequency. Despite individual variation in study designs, the evidence presented suggests that gemcitabine-cisplatin is effective in patients from a diverse range of countries and with heterogeneous disease characteristics. No substantial differences in toxicity were observed among the different dosing schedules of gemcitabine and cisplatin.
IntroductionBiliary tract cancer (BTC) refers to a group of cancers of the biliary tract, including gallbladder cancer, cholangiocarcinoma of intrahepatic and extrahepatic bile ducts, and cancers of the ampulla and papilla of Vater [1,2]. Despite its relatively rarity, the incidence of BTC varies widely in different geographic regions, with the lowest incidence rates in Western countries, including the United States and western Europe, and the highest rates in Asia and Latin America [3]. Gallbladder cancer is the most common type of BTC; however, the proportion of BTC tumors that originate in the gallbladder varies geographically [3,4]. Most patients with BTC are diagnosed at a late stage, in part because there are few, if any, specific symptoms [5]. Surgery is the only curative treatment; however, most patients are ineligible for surgery, either because their tumors are unresectable or because they have other comorbidities that preclude surgical intervention [2,5]. The prognosis for patients with advanced (unresectable and/or metastatic) BTC is very poor, and most survive for less than a year after diagnosis [5,6].
Although surgery remains the only curative treatment, chemotherapy can extend survival of patients with BTC [2]. For example, in an early randomized controlled trial (RCT) of chemotherapy in BTC and pancreatic cancer, treatment with 5-fluorouracil and leucovorin, with or without etoposide, increased median survival of patients with BTC to 6.5 months, compared with 2.5 months achieved with best supportive care [7]. Historically, due to the relative rarity of BTC, conduct of clinical studies of potential therapies has been difficult, and physicians have often used chemotherapy regimens that benefit patients with other gastrointestinal cancers, particularly pancreatic cancer. One such chemotherapeutic agent is gemcitabine, which is a standard of care for patients with advanced, unresectable pancreatic cancer [8], and has been approved by the Food and Drug Administration (FDA) as monotherapy for these patients [9]. In pancreatic cancer, additional survival benefit can be achieved by combining gemcitabine and a platinum agent, such as cisplatin; however, combination therapy may be associated with greater toxicity than gemcitabine monotherapy [10].
Evidence of the efficacy of several gemcitabine-based combination therapies, such as gemcitabine-oxaliplatin and combinations involving targeted therapies, in patients with BTC has been reported [2,11-14], with the most substantial evidence reported for gemcitabine combined with cisplatin.
Evidence of the efficacy of gemcitabine-cisplatin combination therapy was initially provided by small observational and retrospective studies [15]. The first major RCT to assess the efficacy and safety of gemcitabine-cisplatin in BTC was the phase 2 ABC-01 trial [16], which was extended into the phase 3 ABC-02 trial, the largest (n=410) RCT in patients with BTC [17]. In the ABC-02 trial, gemcitabine-cisplatin significantly improved overall survival (OS), progression-free survival (PFS), and tumor control rates compared with gemcitabine monotherapy [17]. Based on the ABC-02 trial, gemcitabine-cisplatin combination therapy has rapidly been accepted as the standard first-line treatment for advanced BTC. Currently, gemcitabine-cisplatin is approved as a firstline treatment for BTC in Korea and Chile, and gemcitabine monotherapy is approved in Thailand, Mexico, Ukraine, and Japan.
The ABC-02 trial is the largest RCT conducted to date, although many smaller clinical studies have been conducted in patients with BTC. However, due to the relative rarity of BTC, enrolling an adequate sample size has often limited these prospective studies. In addition, patients with biliary tract obstruction or infection, which are common concurrent conditions, are excluded from most clinical studies. To maximize enrollment, many studies have used broad inclusion criteria, resulting in heterogeneity of patient characteristics, including primary tumor site and cancer stage (i.e., locally advanced vs. metastatic). However, some evidence suggests that the efficacy of chemotherapy may differ for different tumor types and cancer stages. For example, higher response rate has been reported for patients with gallbladder cancer, but OS was lower after chemotherapy compared to patients with other forms of BTC [18]. Similarly, both intrahepatic cholangiocarcinoma (compared with gallbladder and other BTC sites) and metastatic disease (compared with locally advanced disease) have been identified as independent predictors of poor prognosis in patients receiving chemotherapy [19]. However, the possible effect of primary tumor site on prognosis and response to chemotherapy has yet to be confirmed. Another complicating factor in the assessment of gemcitabine-cisplatin efficacy is the range of regimens used. Most recent studies have followed the ABC-02 trial regimen (1,000 mg/m2 gemcitabine and 25 mg/m2 cisplatin administered on days 1 and 8 of a 21-day cycle [17]); however, other regimens have also been used. Thus, questions remain regarding the optimal regimen for maximizing efficacy while minimizing toxicity. Finally, given the higher incidence of BTC in Asia compared with most Western countries, it is important to assess whether the efficacy and safety of gemcitabine-cisplatin in Asian patients is similar to that in patients from other countries.
The primary objective of this systematic review is to present collated evidence from randomized and nonrandomized prospective studies for the efficacy of gemcitabine-cisplatin in patients with advanced or metastatic BTC. We chose to focus on the gemcitabine-cisplatin combination, rather than other chemotherapeutic regimens, because of its current status as the treatment of choice for BTC and because the relatively large number of studies provide an opportunity to explore potential subgroup differences in efficacy and safety. As such, the secondary objectives of the review are to assess whether the efficacy of gemcitabine-cisplatin is influenced by primary tumor site, disease stage, or geographic region, and to present collated evidence from prospective and retrospective studies of toxicities, including whether these toxicities are influenced by the dose or regimen used.
Materials and Methods1. Literature search strategyThe following databases were searched on 5 December 2013: MEDLINE via PubMed (1946-search date); EMBASE (1966-search date); ClinicalTrials.gov results database (2008-search date); and abstracts from American Society of Clinical Oncology, European Society for Medical Oncology (ESMO), ESMO Gastrointestinal Cancer, and European CanCer Organisation conferences (2009-2013). Free-text terms and medical subject heading (MeSH) or EMTREE terms were used where possible to search for gemcitabine (‘gemcitabine’ and ‘Gemzar’), cisplatin (‘cisplatin’), and BTC (‘biliary tract cancer’ and ‘biliary tract neoplasms’), including specific BTC types and tumor sites (‘gallbladder’, ‘bile duct’, ‘papilla of Vater’, ‘ampulla of Vater’, ‘Klatskin’, and ‘cholangiocarcinoma’). Searches were conducted using truncation symbols and Boolean operators (AND, OR) as needed. There were no restrictions on publication type or language, although the search output was restricted to human studies where possible.
2. Eligibility criteriaIncluded publications/studies evaluated patients who received gemcitabine-cisplatin combination therapy, at any dose or regimen, as first-line treatment for advanced and/or metastatic BTC. Study types considered included metaanalyses, systematic reviews, randomized and nonrandomized clinical trials, and both prospective and retrospective observational studies. Full-text publications, abstracts, and ClinicalTrial.gov trials with posted results were eligible for inclusion. Excluded publications included studies not conducted in humans; studies of patients with cancers other than BTC; studies of therapies other than gemcitabinecisplatin (including gemcitabine alone or combined with other agents); studies of gemcitabine-cisplatin used as second-line therapy, as part of chemoradiotherapy, or administered intra-arterially; studies in which data for gemcitabinecisplatin therapy were pooled with data for other therapies; studies that did not report relevant outcomes (e.g., retrospective studies that did not report safety outcomes); and conference abstracts of retrospective studies. Narrative reviews, systematic reviews that did not report original data, case reports, case series, nonclinical letters, editorials, and commentaries were also excluded.
3. Study selection and data extractionThe literature search and screening of titles, abstracts, and, where necessary, full text of all publications retrieved were performed by one person (not an author) using the predefined eligibility criteria. Reference lists of systematic reviews and other relevant publications were hand screened for identification of additional publications. The publications identified for inclusion were reviewed and approved by all authors.
Data collected from the included publications included publication type and year, country of origin, study design, patient characteristics, treatment regimen, and efficacy and safety outcomes. Aspects relating to study quality (e.g., presence/absence and method of randomization, presence/absence of blinding, study population used for analysis) were also assessed.
Efficacy outcome data were extracted from prospective studies only and included OS, PFS, overall response rate (complete response [CR]+partial response [PR]), CR rate, PR rate, stable disease (SD) rate, progressive disease rate, disease control rate (CR+PR+SD), and any other reported efficacy outcomes. Safety outcome data were extracted from all prospective and retrospective studies and included the type, frequency, and severity of toxicities, deaths and discontinuations related to toxicity, and any other reported safety outcomes.
Results1. Literature search resultsA total of 782 potentially relevant publications retrieved from MEDLINE via PubMed and EMBASE were screened for inclusion (Fig. 1). No relevant unpublished trials were identified on ClinicalTrials.gov. Manual screening identified two additional conference abstracts [20,21]. Overall, 16 fulltext publications [16,17,22-35] and 4 abstracts [20,21,36,37] met the eligibility criteria for inclusion (Table 1).
2. Overview of study characteristicsThe included studies were conducted in a broad range of countries from North America, Europe, Australia, Asia, and Africa (Table 1). Most studies included participants with Eastern Cooperative Oncology Group performance status of 0 or 1, and with primary tumors from various sites. Sample sizes ranged from 10 [21] to 410 [17] participants; 912 participants received gemcitabine-cisplatin. In almost all studies, gemcitabine was administered intravenously at a dose of 1,000 to 1,250 mg/m2 on day 1 and day 8 of a 21-day cycle. The dose of cisplatin was more variable, ranging from 20 to 80 mg/m2, and was usually administered either once (day 1) or twice (days 1 and 8) per cycle.
Of the 17 publications of prospective studies (Table 1), four described open-label RCTs [16,17,27,32]. The ABC-01 [16], ABC-02 [17], and BT-22 [32] trials compared gemcitabinecisplatin with gemcitabine monotherapy, whereas the fourth RCT compared gemcitabine-cisplatin with S-1 plus cisplatin [27]. All RCTs used the intention-to-treat population for efficacy analyses; however, only the ABC-02 trial publication specified the allocation method used for randomization (centralized telephone system). One abstract which described a meta-analysis of the ABC-02 and BT-22 trials [37] was published in full after conduct of our literature search [38]. The 12 remaining publications described nonrandomized, prospective studies [20,21,23,25,26,28-31,33,34,36], of which none were comparative and most included fewer than 50 participants. Of the three retrospective studies that reported safety outcomes, one compared gemcitabine-cisplatin with all other treatments [24], whereas two studies were not comparative [22,35].
3. Efficacy outcomes1) OverallThe most common efficacy outcomes reported in publications of prospective studies were OS and response rates (Table 2, Fig. 2). One abstract [21] which reported conflicting response rates is not included in Table 2 or Fig. 2. Median OS ranged from 4.6 months (reported as 20 weeks) [23] to 11.7 months [17] and overall response rates ranged from 17.1% [30] to 36.6% [23] (Fig. 2). Interestingly, the study reporting both the lowest median OS and the highest response rate consisted exclusively of participants with gallbladder cancer [23]. Disease control rates ranged from 45.7% [30] to 81.4% [17] (Table 2). In the ABC-02 trial, significantly greater OS, PFS, and disease control rate were observed (p < 0.001 for OS and PFS; p=0.049 for disease control rate) in the gemcitabinecisplatin group compared with the gemcitabine only group [17]. In the BT-22 trial, despite numerically better OS, PFS, response rate, and disease control rate in the gemcitabinecisplatin group compared with the gemcitabine group, the differences were not statistically significant [32]. This finding may simply reflect the smaller sample size of the BT-22 trial (n=83) compared with the ABC-02 trial (n=410). However, in a meta-analysis of these two RCTs, significantly greater OS and PFS were observed (p < 0.001) in the gemcitabinecisplatin group than in the gemcitabine only group [37]. In the RCT by Kang et al. [27], no significant differences in OS or PFS were observed between gemcitabine-cisplatin and S-1 plus cisplatin groups.
2) Exploratory subgroup analysesThe percentage of participants with gallbladder cancer varied from 0% [29] to 100% [23], although in most studies, the percentage was between 30% and 50% (Table 1, Fig. 3A). Subgroup analyses of efficacy based on primary tumor site were performed in three studies; however, no statistical comparison between tumor site groups was performed. In the ABC-02 trial [17], there was no difference in treatment effect relative to gemcitabine monotherapy on OS between participants with gallbladder, intrahepatic, extrahepatic, hilar, or ampulla tumors. However, the response rate of participants with gallbladder cancer (37.7%; 23 of 61) was numerically higher than for those with other primary tumor sites (19.0%; 19 of 100). In the BT-22 trial [32], the median OS was numerically lower (9.1 months) in participants with gallbladder cancer compared to those with other primary tumor sites (13.0 months). In a non-randomized trial [30], participants with gallbladder cancer showed a numerically higher response rate (28.6%; 4 of 14) than those with other primary tumor sites (9.5%; 2 of 21). Among all of the studies, there was no apparent relationship between the percentage of participants with gallbladder cancer and OS (Fig. 3A), response rate (Fig. 3A), or disease control rate.
Where reported, the percentage of participants with metastatic disease ranged from 53% [25] to 91% [30] (Table 1, Fig. 3B). The ABC-02 trial found no difference in the treatment effect relative to gemcitabine monotherapy on OS between participants with locally advanced disease and those with metastatic disease [17]. However, a lower hazard ratio was observed in participants with locally advanced disease (0.47; 95% confidence interval [CI], 0.29 to 0.74) compared to those with metastatic disease (0.74; 95% CI, 0.57 to 0.95). Among all of the studies, there was no apparent relationship between the percentage of participants with metastatic disease and OS (Fig. 3B), response rate (Fig. 3B), or disease control rate.
The diversity of countries in which the included studies were conducted enabled comparison of the efficacy of gemcitabine-cisplatin in participants from Asian countries [23,27-30,32-34,36] with that in participants from Western or other non-Asian countries [17,20,25,26,31]. However, no apparent relationship was found between the study region and OS or response rate (Fig. 4).
4. Safety outcomes1) OverallAll included publications reported safety outcomes, except for an abstract that reported a meta-analysis of efficacy results [37]. Most publications reported grade 3/4 hematologic and nonhematologic toxicities (Table 3), and many also reported lower grade toxicities and/or treatment-related deaths and discontinuations. The incidence of the most commonly reported grade 3/4 hematologic toxicities varied widely (anemia, 2.4%-36%; neutropenia, 1.73%-56.1%; thrombocytopenia, 0%-39.0%). The most commonly reported grade 3/4 nonhematologic toxicities were nausea and vomiting, with incidence ranging from 0% to approximately 30%. Few treatment-related deaths (n=5 of 526 participants in studies reporting deaths; 1.0%) or discontinuations due to toxicities (n=55 of 427 participants in studies reporting treatment-related discontinuations; 12.9%) were reported. However, not all studies explicitly reported deaths or discontinuations.
Despite the limitations associated with retrospective studies, safety data from these studies were included in order to maximize retrieval of toxicity-related information. As indicated, only three retrospective study publications met our inclusion criteria [22,24,35]; the incidence of treatmentrelated toxicities in these studies varied widely, as did those in the prospective studies (Table 3).
2) Exploratory subgroup analysesGemcitabine at a dose of 1,000 to 1,250 mg/m2 (Table 1) was used in most included studies, although one abstract reported the use of 300 mg/m2 gemcitabine [21]. There was no apparent relationship between gemcitabine dose (1,000 mg/m2 vs. 1,200-1,250 mg/m2) and the incidence of grade 3/4 anemia, neutropenia, and thrombocytopenia (Fig. 5A), or between gemcitabine dose and the incidence of nausea, vomiting, or other nonhematologic toxicities.
Where reported, cisplatin was administered once (usually on day 1) or twice (usually on days 1 and 8) per cycle (Table 1). In most studies in which cisplatin was administered twice per cycle, lower doses (20-30 mg/m2) were used, compared with studies in which cisplatin was administered once per cycle (60-75 mg/m2). There was no apparent relationship between the frequency of cisplatin administration and the incidence of grade 3/4 anemia, neutropenia, and thrombocytopenia (Fig. 5B), or between cisplatin frequency and the incidence of nausea, vomiting, or other nonhematologic toxicities.
Only five studies reported dose intensities of gemcitabine and cisplatin, most commonly as relative dose intensities (RDIs) [23,28-30,33]. The RDI for gemcitabine ranged from 77.8% to 95%, and the RDI for cisplatin ranged from 78.6% to 99% (Table 1). Given the small number of studies that reported dose intensity, its relationship with either efficacy or safety outcomes remains to be determined.
DiscussionThis systematic review presents collated evidence for the efficacy and acceptable safety profile of gemcitabine-cisplatin combination therapy for treatment of advanced BTC. Seventeen publications of prospective studies and three publications of retrospective studies, involving a total of almost 1,000 participants, were summarized. Despite heterogeneity in study design, disease characteristics, and treatment regimen, the results from these studies provide a large evidence base supporting the efficacy and safety profile of gemcitabine-cisplatin in patients with advanced BTC. Although the ABC-02 trial was pivotal in establishing the efficacy of gemcitabine-cisplatin [17], the collective results of the observational studies included in this review confirm that gemcitabine-cisplatin is effective in patients from diverse countries and with heterogeneous disease characteristics.
Our review provides collective evidence for the beneficial effects of gemcitabine-cisplatin on survival and tumor response in patients with BTC. Although not directly compared in a RCT, the results from prospective studies suggest that gemcitabine-cisplatin extends OS (approximately 5 to 12 months) compared with best supportive care (historically 2.5 to 4.5 months [7,13,18]). In addition, a sizeable proportion of participants in these studies responded to treatment, with tumor response rates ranging from 17.1% to 36.6% and disease control rates ranging from 45.7% to 81.4%. However, because only the four RCTs prospectively compared gemcitabine-cisplatin with other treatments [16,17,27,32], direct comparison with other chemotherapy options is limited. Compared with gemcitabine monotherapy, gemcitabine-cisplatin was associated with longer survival (OS and/or PFS) and greater response and disease control rates [16,17,32,37,38]. One retrospective study (n=85) reported no significant difference in OS or disease control rate between gemcitabine-cisplatin and all other chemotherapy regimens (gemcitabine- or capecitabine-based) [24]. However, this finding should be interpreted with caution, given the retrospective nature of the study and the inclusion of triple therapies involving gemcitabine-cisplatin within the other chemotherapy group. As the range of potential combination therapies expands, including gemcitabine paired with other platinum agents such as oxaliplatin [12,39] and carboplatin [40,41] or with targeted agents such as erlotinib [12], high-quality, prospective, comparative trials similar to the ABC-02 trial are needed for assessment of the relative efficacy of different treatments.
The prognosis for patients with advanced gallbladder cancer is worse than for patients with BTC originating at other sites, with a median survival of 2.8 months if untreated, compared with 5.5 to 10.1 months for untreated cholangiocarcinoma [42]. A pooled analysis of 104 trials (3 of which were included in this review), published in 2007, suggested that patients with gallbladder cancer respond differently to chemotherapy than those with cholangiocarcinoma [15]. Patients with gallbladder cancer (n=500) showed a higher response rate to any chemotherapy (median, 35.5% vs. 17.7%; p=0.008), but a shorter OS (median, 7.2 months vs. 9.3 months; p=0.048), than patients with cholangiocarcinoma (n=471); disease control rate did not differ between the tumor site groups. In contrast, an analysis of participants enrolled in two phase 2 studies or a retrospective cohort study conducted in South Korea identified intrahepatic cholangiocarcinoma as an independent negative prognostic factor for survival [19]. Based on these observations, we explored whether the efficacy of gemcitabine-cisplatin differed depending on the primary tumor site. In the three included studies in which subgroup analyses were performed, response rates tended to be higher [17,30], and OS shorter [32], in participants with gallbladder cancer than in those with other primary tumor sites, which is in agreement with the 2007 pooled analysis for all chemotherapies [15]. However, statistical analysis of the differences between tumor site groups was not performed (presumably because studies were underpowered for subgroup analysis). Consistent with these findings, of all the studies included in this review, the only study that exclusively enrolled participants with gallbladder cancer reported the lowest median OS (20 weeks; 4.6 months) and the highest response rate (36.6%) [23]. A formal, pooled analysis of the relationship between tumor site and efficacy from the studies included in this review was not possible because of study heterogeneity and lack of subgroup data. Based on informal assessment, there was no apparent relationship between the percentage of participants with gallbladder cancer and survival, response rate, or disease control rate. Elucidation of any dependence of efficacy on primary tumor site would require either a prespecified analysis of a large prospective study or a pooled analysis of subgroup-level data from completed studies. Such an analysis, however, would be complicated by the histological heterogeneity even within anatomical tumor sites, as described in recent guidelines on intrahepatic cholangiocarcinoma [43].
We also explored whether the presence or absence of metastatic disease was related to the efficacy of gemcitabinecisplatin. In a phase 3 trial comparing gemcitabine-cisplatin with gemcitabine monotherapy in patients with advanced pancreatic cancer, metastatic disease was identified as a predictor of poor survival [44]. Metastatic disease has also been identified as a predictor of poor outcomes in patients with BTC [19]. Among the studies included in this review, only the ABC-02 trial analyzed the efficacy of gemcitabinecisplatin in participants with or without metastatic disease. Although the advantageous response to gemcitabinecisplatin compared with gemcitabine monotherapy did not differ between groups, a lower hazard ratio was observed in participants with locally advanced disease, suggesting a greater benefit of combination therapy in these participants than in those with metastatic disease [17]. The proportion of participants with metastatic or locally advanced disease was reported in approximately two-thirds of the prospective studies included in this review. However, the available data were insufficient for performance of any formal analysis of the relationship between metastatic disease and efficacy. Once again, we did not identify any clear relationship between the percentage of participants with metastatic disease and survival or response outcomes.
Given the geographic variations in the incidence and type of BTC, it is important to examine any differences in the efficacy of gemcitabine-cisplatin among patients from different geographic regions. We were particularly interested in whether the response of patients from Asia, where both the overall incidence of BTC and the proportion of extrahepatic cholangiocarcinoma are high compared with most Western countries [3], to gemcitabine-cisplatin was similar to that of other patients. In general, similar OS and response rate were reported in studies conducted in Asia [23,27-30,32-34,36] and in Western and other non-Asian countries [17,20,25,26,31]. This observation is supported by comparison of the British ABC-02 [17] and Japanese BT 22 [25] RCTs, which reported similar median OS (11.7 and 11.2 months) and treatment effects of gemcitabine-cisplatin compared with gemcitabine monotherapy (OS hazard ratios of 0.64 [95% CI, 0.52 to 0.80] and 0.69 [0.42 to 1.13]). Collectively, this evidence suggests that gemcitabine-cisplatin is equally effective in both Asian and non-Asian patients with BTC.
In general, the reported toxicities associated with gemcitabine-cisplatin therapy were acceptable and manageable, although the incidence rates varied widely. Of particular importance, few deaths related to treatment were reported, and the overall rate of discontinuation due to toxicity was low (12.9%). In addition, no relationship was found between gemcitabine dose or cisplatin frequency and the incidence of the most common hematologic (anemia, neutropenia, thrombocytopenia) and nonhematologic (nausea, vomiting) toxicities. Although cisplatin administered at lower doses twice per cycle is intended to reduce potential toxicity, use of this approach did not result in any apparent decrease in the incidence rates of toxicities, at least among the studies examined. However, the relatively low number of studies and the differences in study design, participant characteristics, and other aspects of treatment restricts our ability to draw any conclusions. Given the lack of any clear relationship between treatment regimen and toxicity, clinicians may choose to simplify dosing by administering cisplatin at a higher dose once per cycle or to follow the ABC-02 regimen and adjust dosing as needed if high-grade toxicities occur.
As with all systematic reviews, our review is limited by the quality of the studies available for inclusion. Only four RCTs on the use of gemcitabine-cisplatin in advanced BTC have been published, and only one of these was a large, phase 3 trial. All RCTs were open-label by necessity, given the different treatment regimens involved. Most of the other included studies were nonrandomized and uncontrolled, with small sample sizes, reflecting the relative rarity of BTC. To increase recruitment, most studies included participants with different tumor origins and disease stages, resulting in heterogeneous study populations. Unfortunately, the data as presented did not allow performance of any pooled subgroup analyses of the effects of primary tumor site or disease stage. Separate analysis of specific subgroups would require larger sample sizes (e.g., through multinational, collaborative trials) or pooled subgroup-level data, but may reveal differences in the efficacy of gemcitabine-cisplatin in patients with specific disease characteristics. In addition, some studies excluded from this review reported combined data for different chemotherapeutic treatments (e.g., gemcitabine in combination with any platinum agent) or disease (e.g., pancreatic cancer), which, if reported separately, might have been useful in the assessment of gemcitabine-cisplatin in BTC. Reporting of outcomes also varied; for example, not all studies reported deaths or discontinuations. A wide range of treatment regimens were used in the included studies and none of the studies specifically examined the effect of treatment regimen on efficacy or safety/tolerability. Ideally, chemotherapy regimens should be tailored for optimal efficacy balanced with manageable levels of toxicity, and a more systematic assessment of different treatment regimens may indicate which factors (e.g., dose, frequency, cycle length) are most critical to efficacy or tolerability. Finally, there are many other clinically important issues requiring further exploration, but were not the focus of the current review. These issues include optimizing gemcitabine-cisplatin therapy in individual patients (such as those with inadequate biliary drainage), the role of new, targeted agents as adjuncts to standard chemotherapy, and strategies to facilitate conduct of future clinical trials of this rare, but aggressive, family of cancers.
ConclusionIn conclusion, this systematic review presents collective evidence from a range of study designs that supports the use of gemcitabine-cisplatin combination therapy as standard treatment for advanced or metastatic BTC. However, detailed information regarding the effectiveness of gemcitabine-cisplatin in different types of BTC, or toxicities associated with different regimens, is lacking, in part because of the difficulty of conducting studies of sufficient sample size. Of particular importance, despite heterogeneity in the study designs, no substantial difference in toxicity was observed among the different dosing schedules of gemcitabine and cisplatin. In lieu of a large, multinational, collaborative RCT powered to enable subgroup analyses, a meta-analysis of patient-level data could help to address these questions. Alternatively, individual research teams conducting smaller studies should report subgroup-level data, which could facilitate future pooled analyses.
Conflicts of InterestEli Lilly and Company, manufacturer/licensee of gemicitabine (Gemzar), was involved in the study design, data collection, data analysis, and preparation of the manuscript. Do-Youn Oh has received research funding from Eli Lilly. Jen-Shi Chen has received consultancy fees and honoraria from Eli Lilly, Roche, and Novartis. Li-Tzong Chen has received honoraria from Eli Lilly, Novartis, TTY Biopharm, and PharmaEngine, and support for investigator-initiated trials from Merck Serono, Novartis, Sanofi-Aventis, and TTY. Jong Seok Kim is an employee of and owns stock in Eli Lilly Korea Ltd., Republic of Korea. Mauro Orlando is an employee of and owns stock in Eli Lilly Interamerica, Argentina. Joon Oh Park, Chiun Hsu, and Ho Yeong Lim have no conflicts of interest to declare. AcknowledgmentsThe authors thank Chao Zhu, PhD, of Lilly Suzhou Pharmaceutical Co. Ltd., Shanghai, China, and Helen Barraclough, MSc, of Eli Lilly Australia, Ltd., for advice and helpful discussions regarding the scope of this review. Medical writing assistance was provided by Rebecca Lew, PhD CMPP, and Mark Snape, MB BS, of ProScribe – part of the Envision Pharma Group, and was funded by Eli Lilly. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP2).
Fig. 1.Publication flow diagram. ASCO, American Society of Clinical Oncology; BTC, biliary tract cancer; ECCO, European CanCer Organisation; ESMO, European Society for Medical Oncology; gem/cis, gemcitabine-cisplatin therapy; GI, gastrointestinal. 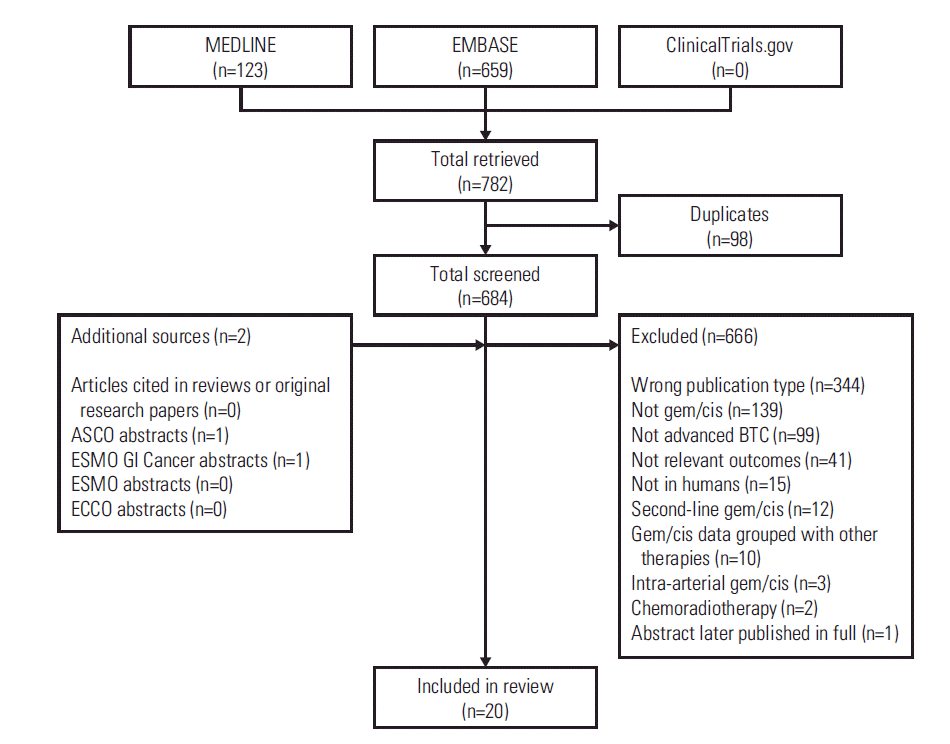
Fig. 2.Forest plots of median overall survival (A) and overall response rate (B) reported in individual publications of prospective studies. Error bars represent 95% confidence intervals (CI; where reported). The number of participants treated with gemcitabine-cisplatin in each study is shown in parentheses. 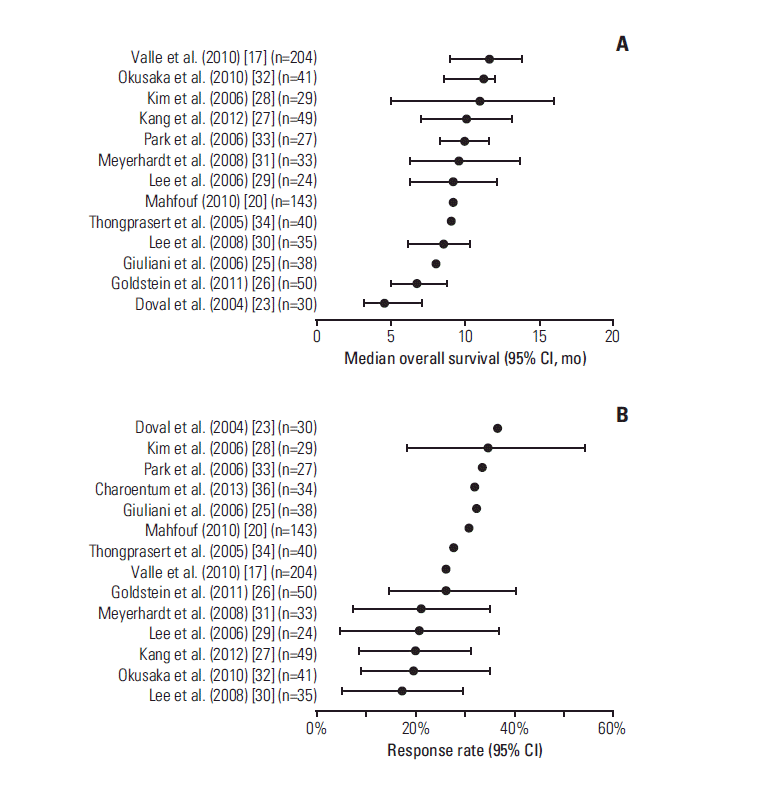
Fig. 3.Forest plots of median overall survival and response rate plotted against the percentage of participants with gallbladder cancer (A) and metastatic disease (B) reported in individual publications of prospective studies. Error bars represent 95% confidence intervals (CI; where reported). 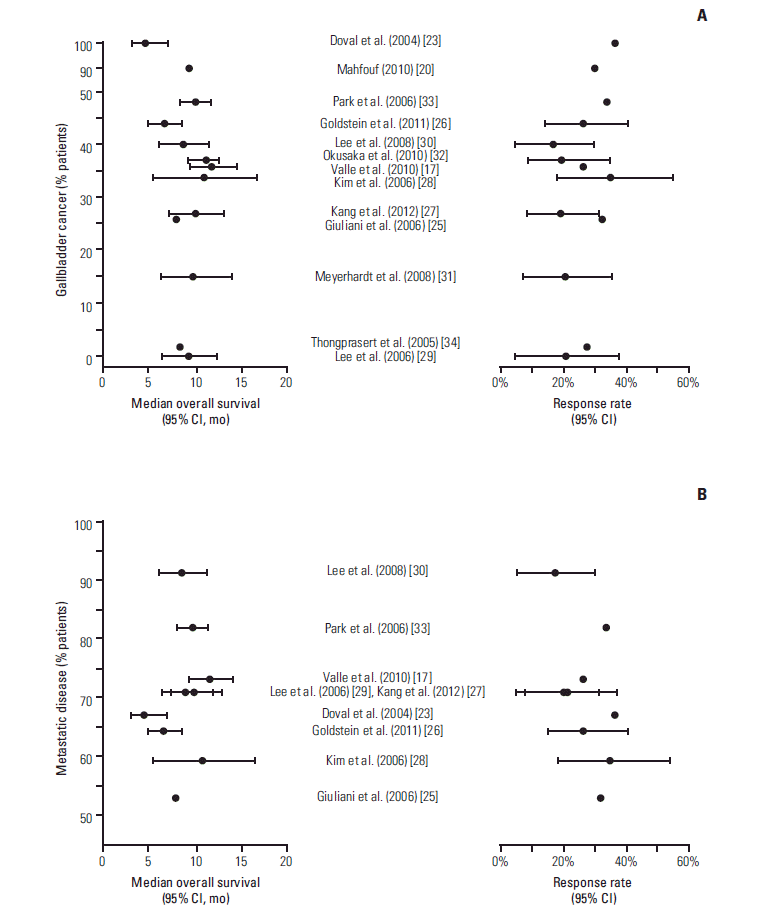
Fig. 4.Forest plots of median overall survival and response rate in individual publications of prospective studies, grouped by geographic region (Western countries vs. Asian countries). Error bars represent 95% confidence intervals (CI; where reported). 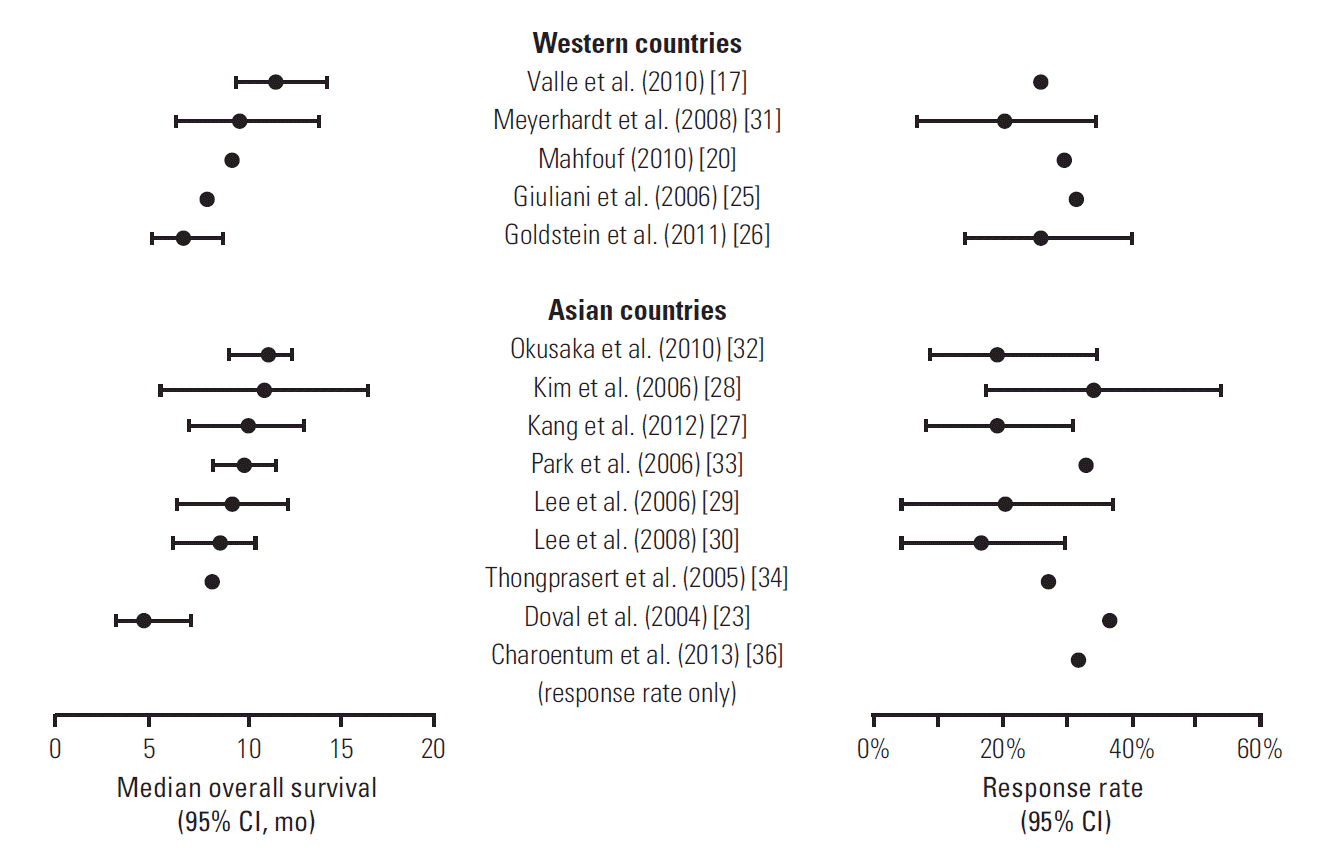
Fig. 5.Incidence of hematologic toxicities in individual publications according to gemcitabine dose (A) and frequency of cisplatin (B). 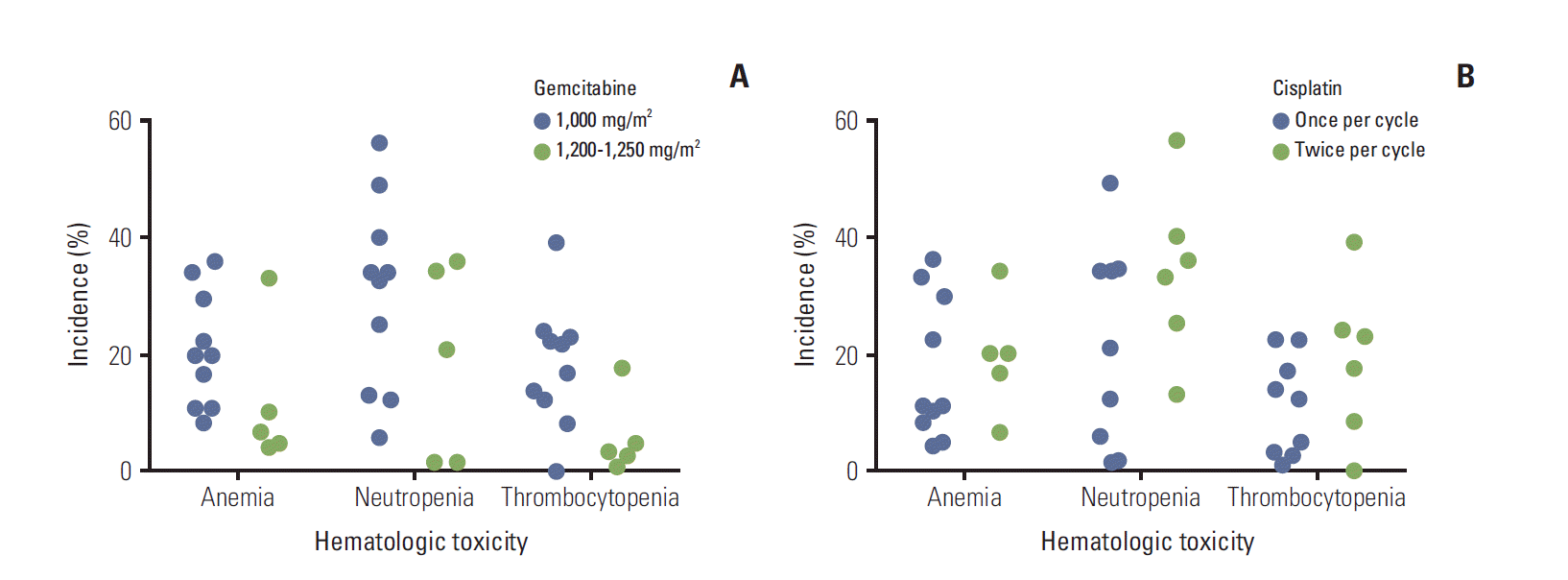
Table 1.Characteristics of included publications on the use of gemcitabine-cisplatin in patients with advanced biliary tract cancer
GC, gemcitabine-cisplatin group; Gem, gemcitabine; NR, not reported; OS, overall survival; PFS, progression-free survival; Cis, cisplatin; GB, gallbladder; CAC, cholangiocarcinoma; PS, performance status; D, day; RR, response rates; TTP, time to progression; RDI, relative dose intensity; TTF, time to treatment failure; SD, standard deviation; BTC, biliary tract cancer. Table 2.Efficacy outcomes of prospective studies of gemcitabine-cisplatin in patients with advanced biliary tract cancer
For comparative studies, data shown are for the gemcitabine-cisplatin group only. OS, overall survival; CI, confidence interval; PFS, progression-free survival; CR, complete response; PR, partial response; DCR, disease control rate; SD, stable disease; PD, progressive disease; NE, not evaluable; NA, not applicable; NR, not reported. Table 3.Safety outcomes of studies of gemcitabine-cisplatin in patients with advanced biliary tract cancer
For comparative studies, data shown are for the gemcitabine-cisplatin group only. Where reported separately, grade 3 and 4 toxicities were added for inclusion in this table. NR, not reported; AST, aspartate transaminase; ALT, alanine transaminase; GGT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; Gr, grade. References1. Noel MS, Hezel AF. New and emerging treatment options for biliary tract cancer. Onco Targets Ther. 2013;6:1545–52.
2. Sasaki T, Isayama H, Nakai Y, Koike K. Current status of chemotherapy for the treatment of advanced biliary tract cancer. Korean J Intern Med. 2013;28:515–24.
3. Randi G, Malvezzi M, Levi F, Ferlay J, Negri E, Franceschi S, et al. Epidemiology of biliary tract cancers: an update. Ann Oncol. 2009;20:146–59.
4. Hundal R, Shaffer EA. Gallbladder cancer: epidemiology and outcome. Clin Epidemiol. 2014;6:99–109.
5. Leonard GD, O'Reilly EM. Biliary tract cancers: current concepts and controversies. Expert Opin Pharmacother. 2005;6:211–23.
7. Glimelius B, Hoffman K, Sjoden PO, Jacobsson G, Sellstrom H, Enander LK, et al. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600.
8. Seufferlein T, Bachet JB, Van Cutsem E, Rougier P; ESMO Guidelines Working Group. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii33–40.
9. Gemzar (gemcitabine for injection) [prescribing information]. Indianapolis: Eli Lilly and Company; 2013.
10. Sun C, Ansari D, Andersson R, Wu DQ. Does gemcitabinebased combination therapy improve the prognosis of unresectable pancreatic cancer? World J Gastroenterol. 2012;18:4944–58.
11. Gruenberger B, Schueller J, Heubrandtner U, Wrba F, Tamandl D, Kaczirek K, et al. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol. 2010;11:1142–8.
12. Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181–8.
13. Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010;28:4581–6.
14. Zhu AX, Meyerhardt JA, Blaszkowsky LS, Kambadakone AR, Muzikansky A, Zheng H, et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11:48–54.
15. Eckel F, Schmid RM. Chemotherapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Br J Cancer. 2007;96:896–902.
16. Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study. The UK ABC-01 Study. Br J Cancer. 2009;101:621–7.
17. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273.
18. Yonemoto N, Furuse J, Okusaka T, Yamao K, Funakoshi A, Ohkawa S, et al. A multi-center retrospective analysis of survival benefits of chemotherapy for unresectable biliary tract cancer. Jpn J Clin Oncol. 2007;37:843–51.
19. Park I, Lee JL, Ryu MH, Kim TW, Lee SS, Park DH, et al. Prognostic factors and predictive model in patients with advanced biliary tract adenocarcinoma receiving first-line palliative chemotherapy. Cancer. 2009;115:4148–55.
20. Mahfouf H. Chemotherapy using gemcitabine (G) plus cisplatin (C) in locally in stage III and IV gallbladder and biliary tract. J Clin Oncol. 2010;28 Suppl:e14654
21. Singh D, Kumar S, Sonkar AA, Jaiswal S, Mishra A. Role of low dose prolonged infusion of gemcitabine and cisplatin in inoperable carcinoma gallbladder: a prospective pilot study. Ann Oncol. 2011;22:v83.
22. Charoentum C, Thongprasert S, Chewaskulyong B, Munprakan S. Experience with gemcitabine and cisplatin in the therapy of inoperable and metastatic cholangiocarcinoma. World J Gastroenterol. 2007;13:2852–4.
23. Doval DC, Sekhon JS, Gupta SK, Fuloria J, Shukla VK, Gupta S, et al. A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer. Br J Cancer. 2004;90:1516–20.
24. Eckmann KR, Patel DK, Landgraf A, Slade JH, Lin E, Kaur H, et al. Chemotherapy outcomes for the treatment of unresectable intrahepatic and hilar cholangiocarcinoma: a retrospective analysis. Gastrointest Cancer Res. 2011;4:155–60.
25. Giuliani F, Gebbia V, Maiello E, Borsellino N, Bajardi E, Colucci G, et al. Gemcitabine and cisplatin for inoperable and/or metastatic biliary tree carcinomas: a multicenter phase II study of the Gruppo Oncologico dell'Italia Meridionale (GOIM). Ann Oncol. 2006;17 Suppl 7:vii73–7.
26. Goldstein D, Gainford MC, Brown C, Tebbutt N, Ackland SP, van Hazel G, et al. Fixed-dose-rate gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol. 2011;67:519–25.
27. Kang MJ, Lee JL, Kim TW, Lee SS, Ahn S, Park DH, et al. Randomized phase II trial of S-1 and cisplatin versus gemcitabine and cisplatin in patients with advanced biliary tract adenocarcinoma. Acta Oncol. 2012;51:860–6.
28. Kim ST, Park JO, Lee J, Lee KT, Lee JK, Choi SH, et al. A Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. Cancer. 2006;106:1339–46.
29. Lee GW, Kang JH, Kim HG, Lee JS, Lee JS, Jang JS. Combination chemotherapy with gemcitabine and cisplatin as first-line treatment for immunohistochemically proven cholangiocarcinoma. Am J Clin Oncol. 2006;29:127–31.
30. Lee J, Kim TY, Lee MA, Ahn MJ, Kim HK, Lim HY, et al. Phase II trial of gemcitabine combined with cisplatin in patients with inoperable biliary tract carcinomas. Cancer Chemother Pharmacol. 2008;61:47–52.
31. Meyerhardt JA, Zhu AX, Stuart K, Ryan DP, Blaszkowsky L, Lehman N, et al. Phase-II study of gemcitabine and cisplatin in patients with metastatic biliary and gallbladder cancer. Dig Dis Sci. 2008;53:564–70.
32. Okusaka T, Nakachi K, Fukutomi A, Mizuno N, Ohkawa S, Funakoshi A, et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103:469–74.
33. Park BK, Kim YJ, Park JY, Bang S, Park SW, Chung JB, et al. Phase II study of gemcitabine and cisplatin in advanced biliary tract cancer. J Gastroenterol Hepatol. 2006;21:999–1003.
34. Thongprasert S, Napapan S, Charoentum C, Moonprakan S. Phase II study of gemcitabine and cisplatin as first-line chemotherapy in inoperable biliary tract carcinoma. Ann Oncol. 2005;16:279–81.
35. Wu CE, Hsu HC, Shen WC, Lin YC, Wang HM, Chang JW, et al. Chemotherapy with gemcitabine plus cisplatin in patients with advanced biliary tract carcinoma at Chang Gung Memorial Hospital: a retrospective analysis. Chang Gung Med J. 2012;35:420–7.
36. Charoentum C, Chotirosniramit A, Chitapanarux T, Sirivanichai C, Lertprasertsuke N, Chewaskulyong B, et al. Attenuated dose of gemcitabine with cisplatin in patients (pts) with unresectable or metastatic biliary tract cancer (ABC): results of single Thai institution. J Clin Oncol. 2013;31 Suppl 4:265.
37. Mizuno N, Valle JW, Furuse J, Jitlal M, Beare S, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomized trials. J Clin Oncol. 2013;31 Suppl:4120.
38. Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, et al. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391–8.
39. Thatikonda C, Miller A, Iyer R. Meta analysis of oxaliplatinbased combination chemotherapy for advanced gallbladder and bile duct cancers (AGBC). Am J Gastroenterol. 2010;105:S72.
40. Julka PK, Puri T, Rath GK. A phase II study of gemcitabine and carboplatin combination chemotherapy in gallbladder carcinoma. Hepatobiliary Pancreat Dis Int. 2006;5:110–4.
41. Williams KJ, Picus J, Trinkhaus K, Fournier CC, Suresh R, James JS, et al. Gemcitabine with carboplatin for advanced biliary tract cancers: a phase II single institution study. HPB (Oxford). 2010;12:418–26.
42. Gallardo J, Rubio B, Villanueva L, Barajas O. Gallbladder cancer, a different disease that needs individual trials. J Clin Oncol. 2005;23:7753–4.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||