INTRODUCTION
The incidence of stomach cancer is decreasing in western countries (1), and it seems to be gradually diminished in Korea. Still, however, stomach cancer is one of the most common cancers in a Korean population (2). The best prognosis can be expected with the treatment of stomach cancer using the early diagnosis followed by radical resection. Unfortunately, however, a great number of patients are diagnosed with stomach cancer after the metastasis was already progressed. Even in the patients who underwent radical resection, the recurrence is still a problem (3).
In patients with stomach cancer in whom recurrence or metastasis were noted, systemic anti-cancer chemotherapy is the only choice. As compared with conservative treatment, the anti-cancer chemotherapy for patients who had a recurrence or a metastasis prolongs a survival and improves quality of life (4,5). Unfortunately, however, the response rate for anti-cancer chemotherapy in patients with stomach cancer amounts to only 30~40% and its median survival does not exceed ten months.
No standard treatment regimens for patients with a recurrence or a metastasis have been established. It has been reported that the combination therapy with ciplatin plus 5-FU rather than 5-FU monotherapy has a higher response rate (6,7). Few evidences have been identified to demonstrate that the combination chemotherapy with cisplatin plus 5-FU prolongs the survival period compared to 5-FU monotherapy (6,7), but it is one of the most popular treatment regimens that are currently used. It is currently well known, however, that the response rate of the combination chemotherapy with cisplatin plus 5-FU amounts to 30~50% and its median survival is at most 7~10 months (6,7).
Within a recent 10-year period, taxane anti-cancer drugs have been frequently used to treat gastric cancer. Docetaxel as a single agent yeilds a response rate of approximately 25% (8), and is commonly used with cisplatin or cisplatin plus 5-FU for the treatment of patients with stomach cancer (9,10).
Paclitaxel showed an approximate response rate of 20~25% and its median duration of response has been reported to be approximately seven months (11-13). Besides, a combination of paclitaxel with cisplatin showed a synergistic effect (14), and this concomitant medication showed a response rate of 33% in patients with stomach cancer including those who previously received anti-cancer chemotherapy (15).
We conducted this study to examine whether the additional use of paclitaxel with the conventional combination chemotherapy with cisplatin plus 5-FU improves the response rate and survival period.
MATERIALS AND METHODS
1) Selection criteria
The current study was conducted in patients who were admitted to department of internal medicine at Inje University Hospital, Sanggye Paik Hospital. Inclusion criteria were 1) age between 18 and 69 years, 2) gastric adenocarcinoma which was histologically or cytologically diagnosed, but could not be treated with radical resection or recurred postoperatively, 3) no past history of anti-cancer chemotherapy or a more than 6-month history of postoperative adjuvant chemotherapy, 4) the presence of measurable lesions, 5) the performance status of ECOG grade 0~2, 6) neutrophil counts of >1,800/mm3 and platelet counts of >100,000/mm3, 7) AST/ALT ≤3×upper normal limits on liver function test, 8) creatinine clearance rate of ≥50 ml/min and 9) submission of a written informed consent.
2) Treatment methods
Paclitaxel 135 mg/m2 was intravenously infused for three hours on day 1. 5-FU was continuously infused intravenously at a dose of 1,000 mg/m2/day for four days from day 1. Cisplatin 60 mg/m2 was intravenously infused on day 1. To prevent vomiting, ondansetron, dexamethasone, metoclopramide and lorazepam were prescribed.
The dose was adjusted based on the following criteria:
1) In cases of grade 2 hepatotoxicity, the dose of paclitaxel for the following treatment was reduced to 110 mg/m2. If the hepatotoxicity was Grade 3 or higher, the study was discontinued.
2) In cases for the bone marrow suppression of Grade 4, the dose of paclitaxel for the following treatment was reduced to 110 mg/m2. If the bone marrow suppression of Grade 4 was persistently present despite the reduced dose of paclitaxel, the study was discontinued.
3) In cases for the mucositis of Grade 3 or higher, 5-FU was administered from the next cycle for three days.
4) In cases for the creatinine clearance rate ranged between 30 and 50 ml/min due to the nephrotoxicity, the dose of cisplatin was reduced by 50%. If the creatinine clearance rate was lower than 30 ml/min, the study was discontinued.
This treatment was repeated at a 3-week interval. In cases in which the evidences demonstrating the progression of lesions were identified, the study was discontinued.
3) An analysis of treatment effect and its statistics
Prior to each treatment, history taking, physical examination, complete blood count, liver function test and a chest radiography were performed. The toxicity of treatment was also evaluated. In cases in which there were no evidences demonstrating the progression of diseases following the treatment, the treatment effect was evaluated using such modalities as an abdominal computed tomography (CT) after each treatment was completed twice. Treatment effect was defined as a complete remission, a partial remission, stable disease and progression based on the RECIST criteria. The adverse effects of the treatment were defined based on the NCI-CTCAE version 3.0.
The primary objective of the current study was to examine the response rate and toxicity. The secondary objectives of the current study were to examine the progression free survival duration, to identify the factors affecting the survival rate and to calculate the overall survival period. An analysis of survival period and the available follow-up period was made using a Kaplan-Meier survival curve.
To demonstrate that the current treatment regimen will elevate a previous response rate of 20% by 20%, with α-error and β-error at 0.05 and 0.10, respectively, the minimax design was used. This showed that the number of patients needed would be 31. Here, considering a drop-out rate of 10%, the total number of patients would be 34.
Statistical analysis was performed using SAS version 4.1.
RESULTS
1) Baseline characteristics
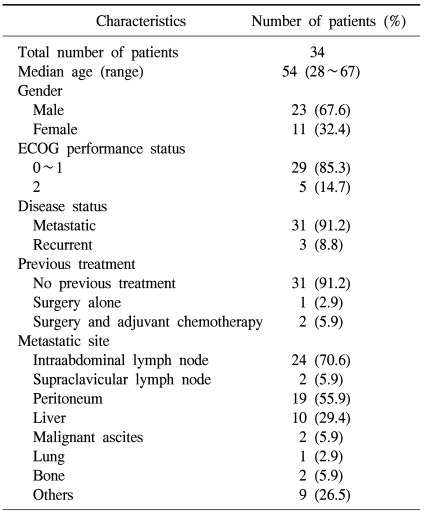
A total of 34 patients pariticipated in the current study between June 2002 and January 2006. The age distribution ranged 28 to 67 years with the median age of 54. The male-to-female ratio was 23:11 and this showed a male predilection. The number of patients with ECOG grade 0-1 and 2 was 29 and 5, respectively. The number of patients who did not undergo surgery due to metastasis was 31 and that of patients who underwent surgery but showed a recurrence was 3. Among the three patients who previously underwent surgery, two received the postoperative adjuvant treatment. The most common site of metastasis was the intraabdominal lymph nodes (Table 1).
2) Dose intensity of treatment drugs
Patients received a total of 178 cycles of treatment. Each patient received the treatment at a median frequency of six times (range 1~9 times). Of the 178 treatments, the planned date for treatment was delayed longer than a week in 27 times (15.2%). The major cause was bone marrow suppression. The dose intensity of each treatment drug was calculated. This showed that the actual administrated amount of paclitaxel, cisplatin and 5-FU were 92%, 91% and 90% of planned doses, respectively.
3) Treatment effect
In 33 of a total of 34 cases, the treatment effect could be evaluated. In one patient, who did not further visit us following one-dose treatment, the assessment of treatment effect was unavailable.
A complete remission was not achieved in 33 evaluable cases, of which 17 achieved a partial remission, 13 showed a stable disease and three showed a progression. This showed a response rate of 51.5% (95% C.I. 26.0~77.0%).
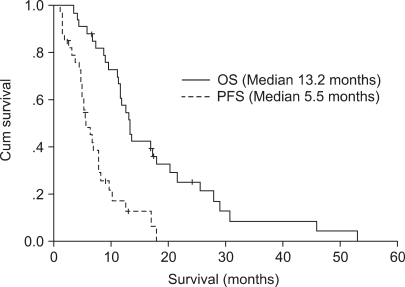
The median values of progression-free survival and overall survival period were 5.5 months (95% C.I. 3.8~7.2 months) and 13.2 months (95% C.I. 11.1~15.3 months)(Fig. 1).
4) Factors affecting the treatment response
An analysis of prognostic factors affecting the treatment response was attempted. Then, a comparison of age, sex, activity, a past history of anti-cancer chemotherapy, the preoperative levels of serum CEA and LDH and the response rate was made between the two groups. On univariate analysis, however, there were no significant differences between the two groups.
5) Treatment-related toxicity
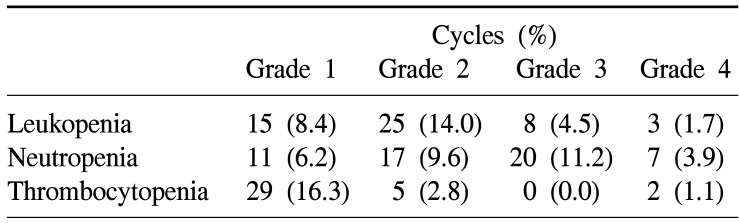
During a total of 178 cycles of treatment, hematologic toxicities of Grade 3 or higher were assessed by the NCI-CTCAE criteria. Leukopenia was developed 11 cases (6.2%); neutropenia 27 cases (15.2%); and thrombocytopenia was developed in 2 cases (1.1%)(Table 2).
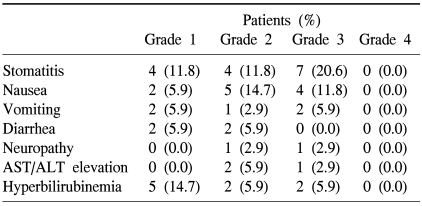
In our series of a total of 34 patients, the hematologic toxicities of Grade 3 include seven cases (20.6%) of stomatitis, four cases (11.8%) of nausea, two cases (5.9%) of vomiting, one case (2.9%) of neurotoxicity, one case (2.9%) of the elevation of AST/ALT and two cases (5.9%) of jaundice. But Grade 4 did not occur. One patient was hospitalized for the treatment of neutropenic fever. But no death cases occurred due to the treatment toxicity (Table 3).
DISCUSSION
To date, the monotherapy with 5-FU as well as the compound chemotherapy with 5-FU has been frequently used for the treatment of patients with metastatic stomach cancer. It has been reported that the combination chemotherapy with cisplatin plus 5-FU increased the response rate compared to the 5-FU monotherapy. But the former is not more effective in prolonging the survival period (6,7).
Taxane anti-cancer drugs, which are commonly used to treat breast cancer, non-small cell lung carcinoma and ovarian cancer, have recently been used for the treatment of patients with stomach cancer.
Recent studies have shown that the additional use of docetaxel to the combination chemotherapy increased the response rate (37% versus 25%) and prolonged a median survival from 8.6 months to 9.2 months (16). In recent years, it has also been reported that the additional use of docetaxel improved the quality of life compared to the combination chemotherapy with cisplatin plus 5-FU. According to this, the concern that the quality of life may be impaired due to the increased toxicity following the administration of the drug has been alleviated (17). Furthermore, the combination therapy with docetaxel, cisplatin plus 5-FU has been reported to have an excellent efficacy in improving the response rate, the survival period and the quality of life compared to that with epirubicin, cisplatin plus 5-FU (18).
A smaller number of studies have examined the efficacy of paclitaxel in patients with stomach cancer as compared with those using docetaxel. One of the studies in this series reported, however, that the combination chemotherapy with paclitaxel, cisplatin puls 5-FU showed a response rate of 51% in patients with metastatic stomach cancer (19).
The current study, where the compound chemotherapy with 5-FU plus cisplatin was combined with paclitaxel, showed a high response rate of 51.5%. This figure is similar to a response rate of 51% which was reported in a previous study (19). This indicates that the additional use of paclitaxel may increase the anti-cancer efficacy.
The current study showed excellent good median survival of 13.2 months. Admitting that the results of a Phase II trial are more excellent than those of a Phase III one, however, the survival period of the combination therapy with docetaxel, 5-FU plus cisplatin does not exceed 12 months in most cases (16,18). Considering this, our results can be regarded as good.
The causes for the excellent survival rate can be explained by the inclusion of relatively younger patients in the current study. In our series, median age of the patients was 55 years was slightly lower than a median age of 55~61 years seen in those who were enrolled in a Phase III clinical trial about the compound chemotherapy with docetaxel, 5-FU plus cisplatin (16,18). In the current study, the organs where stomach cancer was metastasized mostly were the intraabdominal lymph nodes and peritoneum. Once the stomach cancer is metastasized to the extraabdominal space, there are no evidences demonstrating a poorer prognosis than the intraabdominal presence of lesions. It is possible, however, that the number of tumors seen in our patients was smaller than that of those enrolled in other studies. Another cause might originate from the difference in the biological characteristics of stomach cancer between an Asian population and a Caucasian one. On the other hand, the median survival of our series was 13.2 months was relatively longer than usual, considering the median value of progression-free survival of 5.5 months. Most of the patients who were enrolled in the current study further received the secondary treatment if they were refractory to the treatment drugs. To date, no effects of the secondary anti-cancer chemotherapy have been established in patients with stomach cancer. It is plausible, however, that the secondary anti-cancer chemotherapy played a role in prolonging the survival period.
Paclitaxel was added to the compound chemotherapy with 5-FU plus cisplatin in the current study, but the occurrence of hematologic toxicity of Grade 4 was very rare. This finding was contrary to our concern. Presumably, this might be mainly because paclitaxel was used at a low dose of 135 mg/m2. In general, a 3-hour infusion of paclitaxel was used at a dose of 175~225 mg/m2 when it was administered as a single therapy or concomitantly adminsitered with other drugs. It has recently been reported, however, that the combination therapy with a low dose of paclitaxel (145 mg/m2) plus cisplatin was less burdensome to and had an excellent effectiveness for patients with stomach cancer (20). Because we additionally used 5-FU for the current study, we further lowered the dose of paclitaxel to 135 mg/m2. Another reason for the lower hematologic toxicity might be due to the lower incidence of hematologic toxicity observed following paclitaxel treatment than following docetaxel one. On the other hand, it has been reported that paclitaxel causes a higher incidence of neurotoxicity than docetaxel. In the current study, however, neurotoxicity of Grade 3 was noted only in 2.9% of patients. This indicates that the use of paclitaxel at this dose level is safe from the neurotoxicity. In the current study, 5-FU showing much less neurotoxicity compared to the combination chemotherapy with paclitaxel plus cisplatin was added to the treatment modality. It is therefore expected that the current treatment modality has a similar profile of neurotixicity to the combination therapy with paclitaxel plus cisplatin.
To date, various drugs have been used without any clear evidences that demonstrated the effectiveness compared to 5-FU monotherapy in prolonging the survival period in a clinical setting. It can be suggested, however, that the additional use of taxane will establish the standard treatment regimens. Besides, further studies may be necessary to compare docetaxel with paclitaxel in efficacy and toxicity.