AbstractPurposeThe objective of this study was to evaluate external beam radiotherapy (EBRT) in lung cancer patients who suffer from airway obstruction.
Materials and MethodsMedical data of 95 patients with a lung mass that obstructed the airway and received EBRT for it were analyzed. Fifty-nine patients (62.1%) had non-small cell lung cancer and 36 patients (37.9%) had small cell lung cancer. Radiotherapy was given at 8 to 45 Gy (median, 30 Gy) in 1 to 15 fractions (median, 10 fractions). The response to EBRT was assessed through changes in radiographic findings and/or subjective symptoms between before and after EBRT. The median follow-up duration was 124 days. The primary end point was the airway-obstruction resolving rate after EBRT. The secondary end points were patient survival and toxic effects of EBRT.
ResultsImprovement of airway obstruction after EBRT on chest X-ray was achieved in 75 of 95 patients (78.9%). The median time for resolving the radiologic findings and/or symptoms of airway obstruction after EBRT was 7 days (range, 1 to 76 days). The 1-year survival rate was significantly higher in responders than non-responders (12.5% vs. 0.0%, p < 0.001). The biologically effective dose of ≥ 39 Gyα/β=10 (p < 0.01) and the longest obstructive lesion of < 6 cm (p=0.04) were significantly associated with a good response to EBRT in resolving the airway obstruction. No one had grade 3 or higher acute and chronic toxicities.
IntroductionAt the time of diagnosis, the majority of patients with lung cancer are already in an advanced state [1-3], and 50% to 80% of locally advanced lung cancer patients relapse after surgical treatment and/or chemotherapy [4]. When the pulmonary mass progresses so that it obstructs the airway, lung cancer patients often experience dyspnea, cough, hemoptysis, postobstructive atelectasis, pneumonia, and life-threatening conditions [5,6]. These patients usually need prompt treatment to alleviate the agonizing symptoms.
However, metastatic or locally advanced lung cancer patients with airway obstruction have poor performance status. Thus, they are not suitable candidates for chemotherapy or surgery. Immediate management of the airway obstruction is essential to prolong life and improve the quality of life. Endobronchial brachytherapy is widely used for resolving the airway obstruction and is an effective treatment modality for malignant airway obstruction [1,5-12]. However, endobronchial brachytherapy is a time-consuming procedure, and cooperation between physician and patient are essential for effective and safe treatment. Thus, this treatment is impossible for patients with poor performance status or who are unable to cooperate with physicians.
External beam radiotherapy (EBRT) is more available, less time-consuming than endobronchial brachytherapy and can be useful for the treatment of obstructive lesions. However, few trials have been reported for EBRT alone in lung cancer patients with airway obstruction [13-17]. The intent of this study was to assess the efficacy of EBRT for resolving airway obstruction caused by a pulmonary mass.
Materials and MethodsWe reviewed the medical data of 95 patients who had airway obstruction due to lung cancer and underwent EBRT for the obstructive pulmonary mass. Our study protocol was reviewed and approved by Institutional Review Board.
The inclusion criteria were as follows: 1) locally advanced or metastatic lung cancer; 2) radiographic finding of airway obstruction, post-obstructive atelectasis or pneumonia on simple chest X-ray film or computed tomography; and 3) no prior radiation therapy to the chest. Patients were allowed to have prior systemic chemotherapy or resection due to lung cancer. The gross tumor volume included the lung mass, and the conglomerated mediastinal or pulmonary lymph nodes causing the airway obstruction. The gross tumor volume was expanded by a 10-mm radial and 15 to 20 mm craniocaudal margin to create the planning target volume. Respiratory movements were observed under fluoroscopy, and the margins were increased when the target motion exceeded the planned margins. The radiation was delivered to anterior-posterior opposed fields with 6-MV photons.
The response to EBRT was assessed through the changes of radiographic findings and/or subjective symptoms of the patients. Radiologists compared the chest X-ray before EBRT with the chest X-ray after EBRT. The radiologic response was positive when the bronchus that was obstructed before EBRT was opened and a hazy lung field was cleared on follow-up chest X-ray after EBRT. The symptoms of cough, dyspnea, and hemoptysis before and after EBRT were compared and evaluated by radiation oncologists and medical oncologists. However, symptom analyses before and after EBRT by clinicians could be subjective. Thus, airway obstruction improvement is measured by the radiologic response on chest-X rays in this study.
Toxicities were graded by the National Cancer Institute Common Toxicity Criteria ver. 3.0. Grade 3 or higher acute esophagitis, hemoptysis, and radiation pneumonitis were considered meaningful toxic effects. Chronic toxicities such as an esophago-bronchial fistula or pulmonary fibrosis were tracked.
The primary end-point was an airway-obstruction resolving rate after EBRT. The secondary end-points were patient survival and toxic effects of EBRT. Each end-point was measured from the end of the EBRT. All survival rates were estimated by Kaplan-Meier analysis and compared by a log-rank test. Chi-square or Fisher exact test was used to evaluate the significance of the associations between the categorical variables and the tumor response to EBRT. Multivariate analyses were not performed due to the small number of patients with each variable. The biologically effective dose was computed by a linear-quadratic model. Null hypotheses of no difference were rejected if p-values were less than 0.05.
ResultsPatient characteristics are in Table 1. There were 68 men and 27 women, with a median age of 69 years (range, 36 to 85 years). Primary cT3-4 tumors were diagnosed in 77.9% of the patients. The median maximal size of tumors that obstructed the bronchus was 6 cm (range, 2 to 11 cm). At the time of the diagnosis, 64 patients (67.4%) had metastatic disease. Fifty-nine patients (62.1%) had non-small cell lung cancer (NSCLC) and 36 patients (37.9%) had small cell lung cancer (SCLC). Forty-five patients (47.4%) received chemotherapy for metastatic disease before EBRT, and one patient had recurrent disease after surgical treatment of the airway obstruction. Radiation doses of 8 to 45 Gy (median, 30 Gy) in 1 to 15 fractions (median, 10 fractions) were prescribed to the planned target volume. Radiation schedules were as follows: 1) 8 Gy in one fraction, 1 patient; 2) 20 Gy in four fractions, 15 patients; 3) 30 Gy in ten fractions, 74 patients; and 4) 45 Gy in fifteen fractions, 5 patients. The median interval between bronchial obstruction and radiotherapy was eight days (range, 0 to 42 days).
The subjective symptom of airway obstruction was relieved after EBRT in 69 of 95 patients (72.7%). Objective improvement of the airway obstruction on chest X-ray was achieved in 75 of 95 patients (78.9%). All five patients who received radiation of 45 Gy in 15 fractions had a good radiologic response after EBRT. Sixty-five of 74 patients (87.9%) who received radiation of 30 Gy in 10 fractions and five of 15 patients (33.3%) who received radiation of 20 Gy in four fractions had a resolution of obstructed bronchus after EBRT. One patient who received radiation of 8 Gy in one fraction had no radiologic response after EBRT. The median time for resolving the airway obstruction after EBRT was 7 days (range, 1 to 76 days) irrespective of radiation schedules. The airway-obstruction resolving duration after EBRT ranged from 9 to 1,079 days (median, 102 days).
Factors related to the tumor response to EBRT are in Table 2. Age, gender, smoking history, clinical stages, tumor histology, tumor and obstruction site, performance status, interval between bronchial obstruction and radiation, and recurrence were not significantly associated with the tumor response to EBRT. The biologically effective dose (BED) of ≥ 39 Gyα/β=10 (BED of 30 Gy in 10 fractions for early responding tissues) (p < 0.01) and the maximal tumor size of < 6 cm (p=0.04) were significantly associated with a good response to EBRT.
The overall median survival time was 124 days. The median survival time was 201 days (range, 17 to 1,097 days) for the responders to EBRT and 15 days (range, 1 to 200 days) for non-responders. The duration of palliation as a percentage of the survival duration ranged 10.1% to 99.6% (median, 81.1%). The overall survival curve for all patients is seen in Fig. 1. The survival rate for all patients at one year was 9.9%. The 1-year survival rate was significantly higher in the responders to EBRT than non-responders (12.5% vs. 0%, p < 0.001) (Fig. 2).
Among the 75 patients who obtained relief of the airway obstruction, re-obstruction occurred in 33 patients after remission of the obstructive symptoms and signs. Re-irradiation was conducted on 22 patients. The remission of the airway obstruction was achieved again in 18 patients (81.8%) after re-irradiation.
Grade 2 or higher acute toxicities observed during treatment and after irradiation are listed in Table 3. Most acute toxicities were mild to moderate. No grade 3 or higher toxicity occurred in this study. During chest irradiation, grade 2 esophagitis occurred in six of the 95 patients (6.3%). Two patients had grade 2 pneumonitis after chest irradiation and were cured with steroid treatment. There was no esophagobronchial fistula or pulmonary fibrosis due to EBRT in this study.
DiscussionUp to 50% of lung cancer patients suffer from endobronchial lesions, producing uncomfortable symptoms such as dyspnea, hemoptysis, cough, and post-obstructive pneumonia [1,5]. Endobronchial obstruction can be regressed by endobronchial brachytherapy, although complications can develop, including esophago-bronchial fistula, bronchial wall necrosis, bronchitis, and fatal massive hemoptysis [1,7,9,18-20].
Aumont-le Guilcher et al. [12] analyzed the clinical outcomes of 226 patients after high-dose brachytherapy for lung cancer. There was a high rate of complete local response (93.6%), but most tumors (95%) were low stage (Tis to T1) in this study. They found better local disease-free survival in distal tumor locations than proximal locations due to the difficulty in positioning the catheter in proximal tumors. They reported bronchial stenosis in 9.5%, necrosis of the bronchial wall in 3.5%, hemoptysis in 6.6%, and 6% of the patients died of severe complications.
Endobronchial brachytherapy requires specialized facilities and skilled medical teams because it is an invasive procedure and management-related complications sometimes occur, whereas EBRT is a modality that is easily accessible in the community setting [6]. Stout et al. [21] reported a comparative study of endobronchial brachytherapy and EBRT. EBRT was applied at a dose of 30 Gy over 10-12 days using two parallel-opposed fields to cover the tumor, with a margin of 2 cm. Both treatments result in palliation of the symptoms. Statistically significant longer palliation and better survival were found with external beam radiotherapy than with endobronchial brachytherapy, and 51% of the patients who had endobronchial brachytherapy required subsequent external beam radiotherapy for treating recurrent lesions or symptoms after endobronchial brachytherapy. Endobronchial brachytherapy can cover a limited range of endobronchial tumors, due to the abrupt fall-off irradiation outside the radioactive source. However, the treated volume of EBRT should include not only the endobronchial portion of the tumor but also the gross encircling mass which obstructs the bronchus. Thus, EBRT can result in better outcomes for tumor control than endobronchial brachytherapy [21].
Several studies have suggested a concept of the duration of palliation as a percentage of the survival duration [15-17]. The duration of palliation as a percentage of the survival duration was more than 50% in the Medical Research Council (MRC) trial [15]. The range of this index was reported from 28% to 57% (median, 50%) by Lupattelli et al. [16], and from 41% to 96% (median 80%) by Nihei et al. [17]. In our study, the duration of palliation as a percentage of the survival duration ranged 10.1% to 99.6% (median, 81.1%). Thus, our result was comparable with the results of other studies. Higher values of the index were achieved by earlier resolution of the airway obstruction (median, 7 days) in the present study than in other studies, and the lower values can be explained by the patients who had been irradiated with the biologically effective dose of < 39 Gyα/β=10, resulting in re-obstruction symptoms and signs.
In our analysis, EBRT may be a good option to resolve bronchial obstruction; the response rate was 78.9%, and responders to EBRT had a significantly higher survival time than non-responders to EBRT (Fig. 3). It also did not give rise to severe complications. Moreover, in our study, re-irradiation was successfully performed on re-obstructed lesions after EBRT. The airway obstruction resolving rate after re-irradiation was more than 80%. If constraints for normal tissues like the spinal cord, lung, and heart with prior radiotherapy are strictly observed and the dose maintained within the tolerance dose during re-irradiation, this treatment can be performed safely [21].
The maximal tumor size of < 2 cm is a favorable factor to open an obstructed bronchus in lung cancer patients who undergo endobronchial brachytherapy [11,22]. In our trial, the maximal tumor size of < 6 cm was significantly associated with improvement of the bronchial obstruction. Thus, EBRT was more effective for large tumors that obstructed the bronchus than endobronchial brachytherapy.
SCLC follows a more rapid clinical course than NSCLC, and the prognosis is very poor. For limited-stage SCLC, concurrent chemoradiotherapy is a standard treatment. However, definitive chemoradiotherapy is a toxic modality. Thus, for SCLC patients with low performance status, medical comorbidity, and old age, compliance with definitive treatment is low. Moreover, it could be fatal to vulnerable SCLC patients [23-25]. Thus, we recommend palliative radiotherapy, not definitive radiotherapy to limited-stage SCLC patients with bronchial obstruction who are not suited for definitive oncologic treatment due to their low performance status, medical comorbidity, and old age.
ConclusionIn conclusion, EBRT is effective in resolving airway obstruction in lung cancer patients, and the biologically effective dose of ≥ 39 Gyα/β=10 is indicated for opening an obstructed bronchus. Furthermore, the longest obstructive lesion of < 6 cm is expected to have good results with EBRT.
Fig. 2.The 1-year survival rate for responders to the irradiation was significantly higher than non-responders (12.5% vs. 0%, p < 0.001). 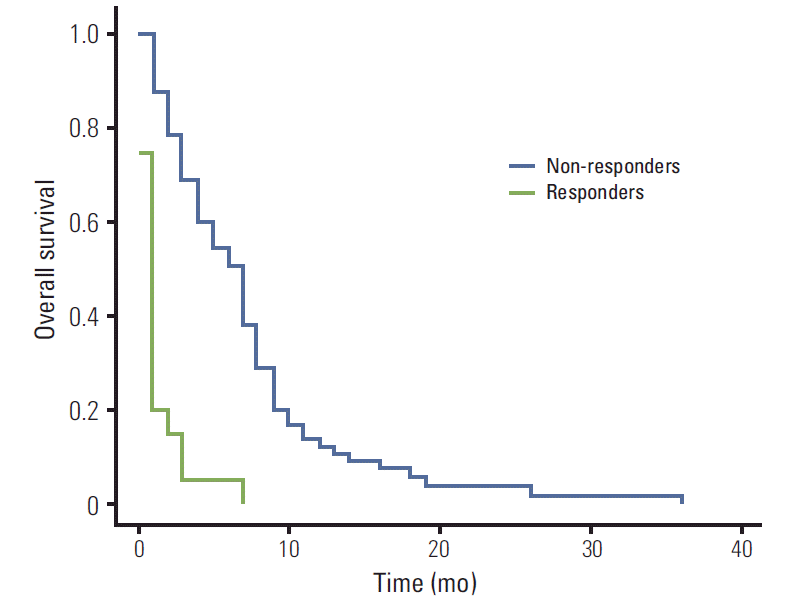
Fig. 3.A patient with small cell carcinoma had obstructive pneumopathy in right upper lobe and received a radiation dose of 30 Gy in 10 fractions. (A) There was an obstructive lesion in right upper lobe at the initial chest X-ray and computed tomography (CT). (B) Radiation-dose distributions in axial and coronal planning CT image. (C) Follow-up chest X-ray and CT showed an improvement of obstruction in right upper lobe 7 days after external beam radiotherapy. 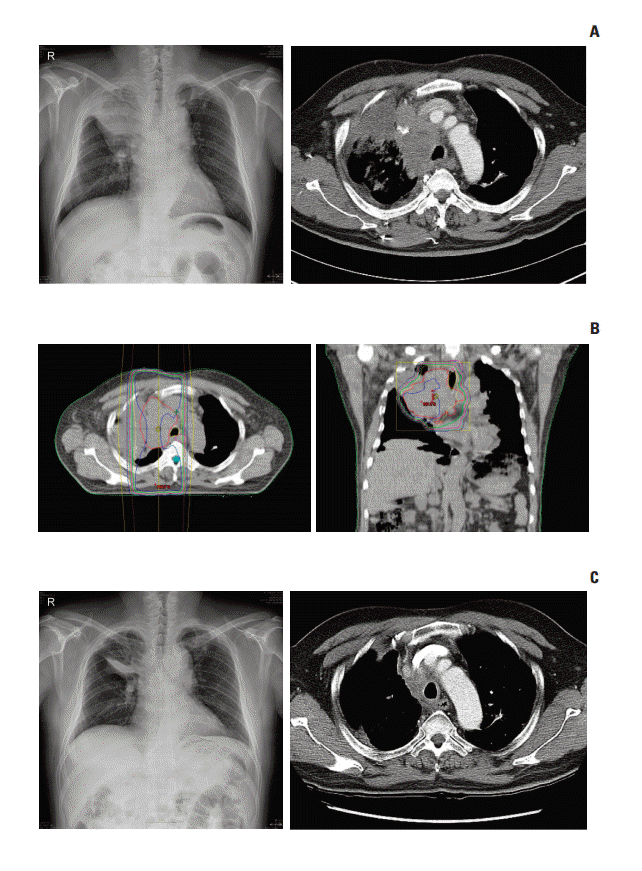
Table 1.Patient and tumor characteristics (n=95) Table 2.Factors associated with improvement of bronchial obstruction
References1. Gollins SW, Burt PA, Barber PV, Stout R. High dose rate intraluminal radiotherapy for carcinoma of the bronchus: outcome of treatment of 406 patients. Radiother Oncol. 1994;33:31–40.
2. Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–62.
3. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–44.
4. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–60.
5. Taulelle M, Chauvet B, Vincent P, Felix-Faure C, Buciarelli B, Garcia R, et al. High dose rate endobronchial brachytherapy: results and complications in 189 patients. Eur Respir J. 1998;11:162–8.
6. Klopp AH, Eapen GA, Komaki RR. Endobronchial brachytherapy: an effective option for palliation of malignant bronchial obstruction. Clin Lung Cancer. 2006;8:203–7.
7. Speiser BL, Spratling L. Remote afterloading brachytherapy for the local control of endobronchial carcinoma. Int J Radiat Oncol Biol Phys. 1993;25:579–87.
8. Zajac AJ, Kohn ML, Heiser D, Peters JW. High-dose-rate intraluminal brachytherapy in the treatment of endobronchial malignancy. Work in progress. Radiology. 1993;187:571–5.
9. Chang LF, Horvath J, Peyton W, Ling SS. High dose rate afterloading intraluminal brachytherapy in malignant airway obstruction of lung cancer. Int J Radiat Oncol Biol Phys. 1994;28:589–96.
10. Macha HN, Wahlers B, Reichle C, von Zwehl D. Endobronchial radiation therapy for obstructing malignancies: ten years' experience with iridium-192 high-dose radiation brachytherapy afterloading technique in 365 patients. Lung. 1995;173:271–80.
11. Harms W, Schraube P, Becker H, Latz D, Herth F, Fritz P, et al. Effect and toxicity of endoluminal high-dose-rate (HDR) brachytherapy in centrally located tumors of the upper respiratory tract. Strahlenther Onkol. 2000;176:60–6.
12. Aumont-le Guilcher M, Prevost B, Sunyach MP, Peiffert D, Maingon P, Thomas L, et al. High-dose-rate brachytherapy for non-small-cell lung carcinoma: a retrospective study of 226 patients. Int J Radiat Oncol Biol Phys. 2011;79:1112–6.
13. Carroll M, Morgan SA, Yarnold JR, Hill JM, Wright NM. Prospective evaluation of a watch policy in patients with inoperable non-small cell lung cancer. Eur J Cancer Clin Oncol. 1986;22:1353–6.
14. Inoperable non-small-cell lung cancer (NSCLC): a Medical Research Council randomised trial of palliative radiotherapy with two fractions or ten fractions. Report to the Medical Research Council by its Lung Cancer Working Party. Br J Cancer. 1991;63:265–70.
15. A Medical Research Council (MRC) randomised trial of palliative radiotherapy with two fractions or a single fraction in patients with inoperable non-small-cell lung cancer (NSCLC) and poor performance status. Medical Research Council Lung Cancer Working Party. Br J Cancer. 1992;65:934–41.
16. Lupattelli M, Maranzano E, Bellavita R, Chionne F, Darwish S, Piro F, et al. Short-course palliative radiotherapy in non-small-cell lung cancer: results of a prospective study. Am J Clin Oncol. 2000;23:89–93.
17. Nihei K, Ishikura S, Kawashima M, Ogino T, Ito Y, Ikeda H. Short-course palliative radiotherapy for airway stenosis in non-small cell lung cancer. Int J Clin Oncol. 2002;7:284–8.
18. Roach M 3rd, Leidholdt EM Jr, Tatera BS, Joseph J. Endobronchial radiation therapy (EBRT) in the management of lung cancer. Int J Radiat Oncol Biol Phys. 1990;18:1449–54.
19. Speiser BL, Spratling L. Radiation bronchitis and stenosis secondary to high dose rate endobronchial irradiation. Int J Radiat Oncol Biol Phys. 1993;25:589–97.
20. Gollins SW, Ryder WD, Burt PA, Barber PV, Stout R. Massive haemoptysis death and other morbidity associated with high dose rate intraluminal radiotherapy for carcinoma of the bronchus. Radiother Oncol. 1996;39:105–16.
21. Stout R, Barber P, Burt P, Hopwood P, Swindell R, Hodgetts J, et al. Clinical and quality of life outcomes in the first United Kingdom randomized trial of endobronchial brachytherapy (intraluminal radiotherapy) vs. external beam radiotherapy in the palliative treatment of inoperable non-small cell lung cancer. Radiother Oncol. 2000;56:323–7.
22. Hennequin C, Bleichner O, Tredaniel J, Quero L, Sergent G, Zalcman G, et al. Long-term results of endobronchial brachytherapy: A curative treatment? Int J Radiat Oncol Biol Phys. 2007;67:425–30.
23. Lee JH, Wu HG, Kim HJ, Kim DW, Lee SH, Kim TM, et al. Influence of comorbidities on the efficacy of radiotherapy with or without chemotherapy in elderly stage III non-small cell lung cancer patients. Cancer Res Treat. 2012;44:242–50.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||