Introduction
Long term dialysis is a risk factor for renal cell carcinoma (RCC) since the prevalence of RCC in hemodialysis or renal transplant patients is 40 to 100 times higher than that of the general population. The kidney and urinary tract organs are susceptible to systemic carcinogenic effects extending from biochemical and immunologic changes that occur with renal pathologies, resulting in loss of proper renal function (1-3).
In spite of increasing numbers of RCC patients with renal impairment, most clinical trials do not include these patients and there is therefore little knowledge regarding systemic chemotherapy in end stage renal disease (ESRD) patients (4). Sunitinib is an oral tyrosine kinase inhibitor (TKI) targeting the vascular endothelial growth factor receptor and the platelet derived growth factor receptor. Given as first-line treatment in metastatic RCC, it has shown clinical activity in a phase III randomized trial and is the current standard treatment for metastatic RCC (5-8). It is usually administered as a once daily dose of 50 mg in a schedule of 4 weeks on and 2 weeks off. Sunitinib and its main metabolite, SU12662, are mainly eliminated in the feces and only 15 to 20% of the dose is cleared by kidney (9). The proper dose and schedule of sunitinib have yet to be established in patients with metastatic RCC on hemodialysis. In addition, long-term treatment outcome and tolerability of sunitinib in patients on hemodialysis have not been reported.
Here, we describe two patients with metastatic RCC on hemodialysis who were treated with intermittent doses of sunitinib in accordance with the patient's renal function.
Case Report
1. Patient
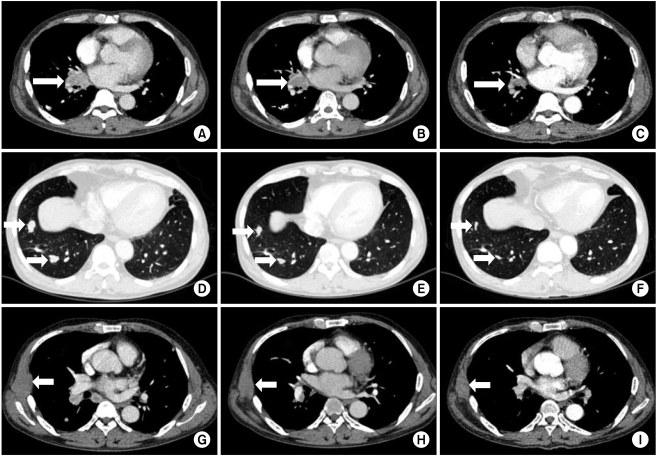
A 53-year old male underwent right nephrectomy for stage II (pT2N0M0) clear cell RCC in 1996. In 2004, a mass was detected in the right gluteus muscle which proved to be metastatic RCC on biopsy. He underwent radiation therapy after which he was confirmed as having no residual lesions. Thereafter he developed impaired renal function with serum blood urea nitrogen (BUN) 85 mg/dL and creatinine 7.5 mg/dL. Hemodialysis was begun three times a week in 2004. In 2005, right lung wedge resection was performed due to a metastatic lesion (Fig. 1A) and he was free of recurrent or metastatic lesions until January 2009 when multiple lung metastases developed. The prognostic risk category of the patient was intermediate as defined by the Memorial Sloan-Kettering Cancer Center (MSKCC) risk model, with Karnofsky performance status 90%, serum hemoglobin 10.7 mg/dL, serum corrected calcium 10.6 mg/dL, lactate dehydrogenase (LDH) 168 IU/L. Baseline computed tomography (CT) scan of the chest showed right hilar lymph node enlargement, a chest wall mass and multiple lung metastases. Fifty milligrams of sunitinib was administered intermittently after each hemodialysis session (3 times a week) from 15 January 2009. The best response was partial response (PR) with 30% size reduction from baseline (Fig. 2). At the time of reporting, April 2010, the patient was tolerating sunitinib relatively well and the progression-free survival (PFS) was 16 months. Most adverse effects observed were neuropathy, facial edema, skin rash, yellowish skin pigmentation, diarrhea, thrombocytopenia, anemia and leucopenia. All of these toxicities were manageable (grade 1). Blood pressure was well controlled with standard anti-hypertensive medications and there was no evidence of hypothyroidism. Although, the patient has been tolerating sunitinib, complications such as increasing chronic fatigue and general weakness, resulting in decreased general performance and activity, were apparent after 1 year of treatment.
2. Patient
A 67-year old female was diagnosed with ESRD due to hypertension and started hemodialysis in May 2006. After 1 month of hemodialysis, a left kidney tumor developed and she underwent nephrectomy for stage I (pT1N0M0) clear cell RCC. In May 2009, multiple metastatic lesions began to develop in her right auricular, right forearm, right axillary, and right scapular areas. She also developed additional lymph nodes at her right hilum and multiple metastatic lesions in her lungs and bones. Biopsies of her right auricular and forearm masses showed metastatic clear cell RCC (Fig. 1B). The prognostic risk category of the patient was intermediate as defined by the MSKCC risk model with Karnofsky performance status 90%, serum hemoglobin 11.4 mg/dL, serum corrected calcium 10.7 mg/dL, LDH 229 IU/L. Fifty milligrams of sunitinib was administered intermittently after each hemodialysis session (4 times a week) from November 2009. The best response was PR with 34% size reduction from baseline after 4 months of treatment (Fig. 3). Adverse events such as aggravation of hypertension or hypothyroidism did not occur. At the time of reporting, April 2010, she was tolerating intermittent sunitinib well and the PFS was 6 months.
Discussion
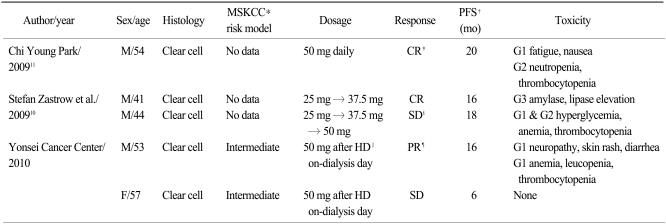
We compared our cases with 3 previously reported cases (in 2 reports) in which metastatic RCC patients on hemodialysis were treated with sunitinib (Table 1) (10,11). All cases, including ours, involved clear cell type RCC. The prognostic risk categories of the patients in our report were intermediate as defined by the MSKCC risk model, but there were no such descriptions in the previous reports. There were similarities in the responses and toxicities of the previous reports and ours. However, the dosage methods were different in each report. Sunitinib was administered daily in 6-week cycles: administration for 4 weeks followed by 2 weeks off treatment in both previous reports. The sunitinib dose was 50 mg daily irrespective of hemodialysis in Park's report (11). In contrast, Zastrow et al. (10) started with a sunitinib dose of 25 mg daily, irrespective of hemodialysis, and increased to 37.5 mg and 50 mg according to the patients' tolerance. Overall responses were complete response (CR) by the patient in Park's report (11), and CR by one patient and stable disease by the other patient in Zastrow et al.'s report (10). However, the dose intensity in these two reports was similar to that used with non-ESRD patients, and it seemed that higher cumulative doses of the sunitinib resulted in better responses. Our sunitnib dose intensity, 50 mg after each hemodialysis session (3 or 4 times a week), was much lower than the dose intensities of the previous reports. Both patients had achieved PR at the time of reporting and we expect to see improvements of their outcomes as the cumulative dose increases.
During the planning stages of our sunitinib therapy, we were unable to find any guidelines for treatment of hemodialysis patients. We therefore decided to treat patients with the dosage method described above on the basis of three rationale: 1) the half-life of sunitinib is 40 to 60 hours; 2) reduced doses of usual cytotoxic drugs are used in hemodialysis patients (4); 3) long-term tolerability sustains durable response, especially with TKI treatments in RCC. Both subjects tolerated the treatment without interruptions of sunitinib. Significantly, the time to response (1 year) was longer than that seen with other cytotoxic drugs or conventional sunitinib. Thus, it is important to keep patients tolerating treatment in order to maintain prolonged response. Furthermore, Hong et al. (12) reported that sunitinib toxicities accumulated and were apparent after approximately 1 year of sunitinib treatment in more than 50% of patients. Therefore, standard dose long-term sunitinib treatment might aggravate underlying hypertension and general weakness of ESRD patients. It is thus important to find an effective dosage associated with minimal toxicity. In this regard, our dosage method would have the same efficacy in metastatic RCC patients on hemodialysis as previous reports and we suggest that it be widely adopted.
From our experiences with two patients, we suggest that the intermittent treatment mode used here could be an alternative option for treating metastatic RCC in the context of hemodialysis: fifty milligrams of sunitinib after each hemodialysis session.