Introduction
High-dose methotrexate (HD MTX) is a major chemotherapeutic agent that is used for the treatment of osteosarcoma [1]. Individual monitoring of the methotrexate (MTX) serum levels enables appropriate alterations of the leucovorin dosage and this can help avoid potential life-threatening toxicities [2]. For example, without leucovorin rescue based on MTX serum level monitoring, the mortality rates due to serious complication range from 4.6% to 6% [2].
Nevertheless, serious adverse events still occur because of delayed MTX excretion. Hemodialysis, hemoperfusion and peritoneal dialysis have all been attempted, but these procedures place cancer patients at risk of other serious complications such as bleeding or infection [3]. Carboxypetidase G2 (CPDG2) shows promise as a means of increasing MTX excretion and a clinical trial for it is underway [4]. However, as yet the safety data has not been made available, and especially for children.
For this reason, the treatment of patients with delayed MTX excretion inevitably depends largely on supportive measures. We report here on two cases of pediatric osteosarcoma in whom the delayed MTX excretion was managed using supportive measures. MTX excretion was promoted in these patients without having to adopt invasive procedures, and any potential life-threatening complications were successfully prevented by administering high doses of leucovorin.
Case Report
1. Case 1
A 4-year-old boy was diagnosed as having osteosarcoma of the left proximal humerus. His laboratory test results, including the serum creatinine and creatinine clearance, were all in the normal range. His first course of HD MTX was administered at 12 g/m2. One hour before MTX infusion, he was intravenously hydrated with 250 mL/m2 of 5% glucose together with containing 100 mol/L NaHCO3 and 20 mL KCl solution. The MTX was dissolved in 500 mL of D5W and infused over 4 hours. After this MTX infusion, he was hydrated with the same fluid to a total of 1,500 mL/m2 during the first 24 hours. His 4-hour MTX level was 1,522 µmol/L, and he suffered from mild nausea. His oral intake was moderate and the urine volume was maintained at above 1,750 mL/day. The following day, he had gained 1.2 kg in weight (range, 19.4 to 20.6 kg), and he complained of abdominal pain, nausea and vomiting. His 24-hour MTX level was 781 µmol/L, and thus, leucovorin was immediately escalated to 1,000 mg/m2 and administered every 3 hours. His serum creatinine level increased up to 2 mg/dL, but his urine output was maintained at above 50 mL/m2/hr without diuretics. He suffered oral mucositis (grade III), and yet he did not experience any diarrhea, fever or leukopenia. Over the following hospital days, his MTX level decreased very slowly and finally it reached to 0.10 µmol/L 10 days after high dose leucovorin rescue (Fig. 1). Following recovery, he proceeded to chemotherapy comprised of cisplatin (100 mg/m2) and doxorubicin (60 mg/m2). The second course of chemotherapy was comprised of a reduced MTX dose (6 g/m2), cisplatin (100 mg/m2) and doxorubicin (60 mg/m2), and it was completed uneventfully. After surgery, he had received all the scheduled chemotherapy, with the full dose of MTX (12 g/m2). He did not suffer any renal complication during postoperative chemotherapy and at present, he has been continuously disease free for 5 years.
2. Case 2
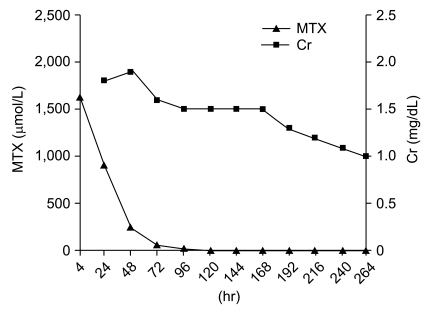
A 7-year-old girl with osteosarcoma of the right distal femur received HD MTX (12 g/m2). She had previously undergone four courses of HD MTX uneventfully at another institution. The laboratory tests revealed asymptomatic microscopic hematuria, and yet her renal function (serum creatinine and creatinine clearance) was normal. She had not been administered any other nephrotoxic agent, such as cisplatin or ifosfamide. HD MTX was administered as described in case 1. During four hours of MTX administration, she suffered nausea and vomiting and the estimated amount of vomitus was about 150 mL. Her MTX level (4-hour) peaked at 1,630 µmol/L. Over the following 20 hours, she continuously suffered from nausea and vomiting. Her 24-hour MTX level was 892 µmol/L and leucovorin was immediately escalated to 1,000 mg/m2 and administered every 3 hours. Her urine output slowly began to decrease and her serum creatinine level increased from 0.4 mg/dL to 1.8 mg/dL (Fig. 2). Diuretics were administered to maintain a urine output of above 50 mL/m2/hr. She complained of abdominal pain, nausea and experienced several episodes of vomiting. However, she did not experience any mucositis, diarrhea or leukopenia. Her serum MTX level decreased slowly, but it finally reached 0.13 µmol/L on the 16th day (384 hours after HD MTX administration). Upon recovery, she proceeded to surgery because her tumor size was increasing. Due to the poor response to preoperative chemotherapy, her postoperative chemotherapy was modified to the regimen comprised of cisplatin (100 mg/m2), doxorubicin (60 mg/m2), ifosfamide (14 g/m2) and bleomycin (30 mg/m2). At present, she has been continuously disease free for 6 years and is still suffering from intermittent hematuria.
Discussion
To the best of our knowledge, our two cases are the youngest reported cases of delayed MTX excretion. Both patients were successfully managed using supportive measures. However, the factors that predisposed the patients to delayed MTX excretion were unclear in these cases. A reduced effective blood volume or the concurrent use of nephrotoxic agents are known to be related to the development of delayed MTX excretion [5,6], but neither patient had signs of decreased blood volume nor they were concurrently receiving any nephrotoxic agent [7]. Furthermore, the previous courses of HD MTX in case 2 and the following chemotherapy (case 1) were uneventful.
The pharmacokinetics of HD MTX shows individual variability [8]. After administration when the renal function is normal, more than 90% of the MTX administered combines with albumin and it is excreted to the urine within 12 hours [9]. The excretion of MTX is influenced by many factors such as age, the hydration status and the concurrent use of nephrotoxic agents [2]. Furthermore, it has been reported that delayed MTX excretion may occur in the absence of any predisposing factor [5,10,11], and in our cases, the reason for delayed MTX excretion was not determined. However, we surmise that a young age or a small body size might have contributed to its development. Given a low body weight, a poor oral intake or severe vomiting during MTX infusion could lead to volume depletion, even with intravenous hydration.
In the past, delayed MTX excretion was largely treated using supportive measures; the key element of which was to increase the leucovorin dosage [2]. However, concern exists that elevated leucovorin dosages might compromise the antitumor effect of MTX [12]. The serum level of MTX is supposed to reflect the drug concentration in tumor cells, and if leucovorin is administered at elevated dosages, there is a possibility that tumor cells might be rescued. Meyers et al. [12] found that the survival of osteosarcoma patients treated with the Children's Cancer Study Group 782 protocol was poorer than that of those treated with the T10 protocol of the Memorial Sloan-Kettering Cancer Center, and they suggested that this might be attributable to the different leucovorin dosages that were administered. On the other hand, it has been reported the expression profiles of reduced folate carrier in normal somatic cells and tumor cells differ, and that as a result, leucovorin targets the normal cells [1]. However, further studies are needed to clarify the effect of leucovorin on tumor cells.
Hemodialysis has been attempted in cases of delayed MTX excretion that fail to respond to supportive measures [13]. However, controversy exists regarding the efficacies of hemodialysis and hemoperfusion. In one literature study, single remedy conventional hemodialysis lowered the serum MTX levels by about 52%, but it took 14 days to achieve complete MTX excretion [2]. Furthermore, it has been reported that high-flux hemodialysis is more effective and removes more MTX than conventional hemodialysis (75.7% vs. 52%) over a shorter period of time (4 hours vs. 14 days) [13-15]. However, rebound remains a problem [2], and the invasive nature of catheter placement may place cancer patients at risk for complications such as bleeding or infection [3].
Various pharmacotherapies have been developed and implemented in cases of delayed MTX excretion. Thymidine directly inhibits the action of MTX, but the continuous infusion of high doses is required because of its short half life [11]. CPDG2 is considered a promising agent that inactivates MTX by hydrolyzing the glutamic acid located at its C-terminal [4]. In one study in twenty-one cancer patients with delayed MTX excretion, CPDG2 reduced the serum MTX levels by 98% within 15 minutes, without any serious adverse reaction [14]. Clinical trials are currently underway [4], and it is hoped that this development will lead to the implementation of HD MTX chemotherapy in an ambulatory setting, which would substantially reduce medical costs.
In conclusion, two pediatric osteosarcoma patients with delayed MTX excretion were successfully managed using supportive measures without any invasive procedure. Potential life-threatening complications were prevented by administering leucovorin at high dosages. Currently, HD MTX chemotherapy requires an extended period of hospitalization and presents the risks of serious complications related to delayed excretion. It is hoped that in the future, CPDG2 will be used to treat patients with delayed MTX excretion and enable HD MTX chemotherapy to be performed on an ambulatory basis.