AbstractPurposeEffectiveness and safety of clofarabine (one of the treatment mainstays in pediatric patients with relapsed/refractory acute lymphoblastic leukemia [ALL]) was assessed in Korean pediatric patients with ALL to facilitate conditional coverage with evidence development.
Materials and MethodsIn this multicenter, prospective, observational study, patients receiving clofarabine as mono/combination therapy were followed up every 4–6 weeks for 6 months or until hematopoietic stem cell transplantation (HSCT). Response rates, survival outcomes, and adverse events were assessed.
ResultsSixty patients (2–26 years old; 65% B-cell ALL, received prior ≥ 2 regimen, 68.3% refractory to previous regimen) were enrolled and treated with at least one dose of clofarabine; of whom 26 (43.3%) completed 6 months of follow-up after the last dose of clofarabine. Fifty-eight patients (96.7%) received clofarabine combination therapy. Overall remission rate (complete remission [CR] or CR without platelet recovery [CRp]) was 45.0% (27/60; 95% confidence interval [CI], 32.4 to 57.6) and the overall response rate (CR, CRp, or partial remission [PR]) was 46.7% (28/60; 95% CI, 34.0 to 59.3), with 11 (18.3%), 16 (26.7%), and one (1.7%) patients achieving CR, CRp, and PR, respectively. The median time to remission was 5.1 weeks (95% CI, 4.7 to 6.1). Median duration of remission was 16.6 weeks (range, 2.0 to 167.6 weeks). Sixteen patients (26.7%) proceeded to HSCT. There were 24 deaths; 14 due to treatment-emergent adverse events.
IntroductionAcute lymphoblastic leukemia (ALL), the most common pediatric malignancy affects around 1 in 1500 children globally, representing 25% of all the pediatric malignancies [1–3]. In Korea, approximately 28 children (age, 0 to 14 years) per million are afflicted by lymphoid leukemia and about 300 new cases of childhood ALL are diagnosed out of 1,500 patients with pediatric cancer every year [4,5].
In new pediatric ALL cases, use of modern treatment strategies such as improved risk stratification based on prognostic factors, newer treatment modalities, and adjustments in treatment regimen intensities have resulted in a 5-year event-free survival rate of approximately 80%, and 5-year survival rate approaching 90%, one of the highest in any pediatric cancers in developed nations [6,7]. However, 15%–20% of patients with pediatric ALL experience relapse during or after the first treatment [8,9]. The primary treatment goal of pediatric relapsed ALL is to achieve secondary remission for a successful hematopoietic stem cell transplantation (HSCT) [10,11]. Despite substantial secondary remission rates (71%–93%) and availability of HSCT, nearly 50% of children with relapsed ALL do not survive [8,9]. In addition to relapsing ALL, the treatment outcomes of pediatric refractory ALL remain a therapeutic challenge [8].
Most of the salvage regimens for relapsed/refractory (R/R) ALL utilize the same frontline therapy agents that may have reached their limits of optimal use and tolerability [12,13]. In such case, novel therapeutic agents such as clofarabine may serve as an effective option. Clofarabine is a second-generation purine nucleoside metabolic inhibitor with better efficacy and toxicity profile than its structural analogues, fludarabine and cladribine that were used to treat hematological malignancies [14]. Clofarabine was approved both in the United States and Europe as a single agent for the treatment of children with ALL refractory to at least two prior regimens [9].
In a retrospective analysis of Korean patients with pediatric R/R ALL (Chopin study), 5-year long-term survival rate was only 6.7%, demanding new treatment options in this group [15]. In 2013, the Korean Ministry of Food and Drug Safety considered clofarabine eligible for reimbursement under the risk-sharing agreement, satisfying the condition of coverage with evidence development (CED), and approved its use in previously treated patients with ALL who had poor prognosis [16]. However, data related to the effectiveness of clofarabine in Korean patients for reimbursement is scarce.
This is the first risk-sharing model for a new drug implemented in a study to generate data in order to execute conditional CED, proposed by the sponsor and approved by Health Insurance Review and Assessment Service in Korea. This study aimed to assess the effectiveness of clofarabine in Korean pediatric patients with R/R ALL, supporting CED. This study is the first clinical outcomes generated to support for the approval of reimbursement from Health Insurance Review and Assessment Service in Korea. Findings from this study will facilitate insurance coverage of patients with ALL who need clofarabine and monitor the long-term outcomes.
Materials and Methods1. Study groupThis multicenter, prospective observational study was conducted at 11 Korean hospitals between May 2014 and January 2018. The study centers where pediatric hematology-oncology specialists practiced with focus on treating pediatric patients with ALL, were chosen. Patients were eligible if they had histologically proven ALL based on the French-American-British Classification, ≥ 25% stem cells in bone marrow, age 1–21 years at the initial diagnosis, and failed to respond or relapsed after ≥ 2 regimens [17]. Eligible patients received clofarabine for the first time after enrollment in the study and had not received other investigational product(s) within 2 weeks prior to enrollment. Patients with severe renal or hepatic impairment, hypersensitivity to the investigational products, pregnant or nursing women were excluded.
2. TreatmentClofarabine was administered in a way of either monotherapy or combinational therapy based on a investigator’s discretion. Clofarabine as a monotherapy was administered intravenously at a dose of 52 mg/m2 over 2 hours daily for 5 consecutive days, at an interval of 2–6 weeks [18]. In the absence of internationally established standard dosing regimen, the dose and duration of clofarabine and other agents (such as cyclophosphamide and etoposide) in the combination therapy regimen were based on the previous studies [19,20]. Although clofarabine was intended to be administered at a dose of 52 mg/m2 over 2 hours daily for 5 consecutive days, the clofarabine dose was reduced for safety reasons in most of the patients. Considering the clinical condition of patients, the dose of clofarabine was adjusted to lower than 52 mg/m2/day in combination therapy. Thus, the most frequent dose of clofarabine was 40 mg/m2/day for the first cycle and the mean dose of clofarabine was 181.9 mg/m2/cycle. HSCT was decided based on clinical response to clofarabine and investigators’ discretion.
3. Data collection during the studyDuring the clofarabine administration, patient data were collected at each clofarabine cycle. After the completion of clofarabine administration, patients were followed up every 4–6 weeks (depending on their disease status) for 6 months or until the day of HSCT, whichever occurred first. Survival status of enrolled patients was collected at the end of study period (3 years from the initiation of the study). Patients were considered to reach the completion of the study when they finished 6 months of follow-up after clofarabine or received HSCT.
Patient demographics, disease characteristics, disease status, response to treatment, safety information, and survival status data were collected at baseline and during the study follow-up visits, as applicable.
4. Response criteria and safetyResponse to treatment was measured by analyzing bone marrow aspirate or biopsy material and confirmed by the investigator. Complete remission (CR) was defined as having bone marrow blasts < 5%; no evidence of circulating blasts or extramedullary disease; absolute neutrophil count > 1.0×109/L; and platelet count > 100×109/L. A complete remission without platelet recovery (CRp) was defined as meeting all CR criteria except for thrombocytopenia (platelet < 100×109/L). Partial remission (PR) was also defined as meeting all hematologic criteria of CR and having the bone marrow blasts between 5% and 25%. The bone marrow assessment for remission was evaluated at the end of each clofarabine cycle and the evaluation of response was done after the last dose of clofarabine. The duration of remission was estimated from the date of last bone marrow evaluation confirming remission after enrollment to the last date of R/R ALL diagnosis/death, whichever occurred first.
Adverse events (AEs) were monitored from the date of signature on informed consent till 30 days after the last clofarabine dosing. AEs were graded by the investigator using National Cancer Institute Common Terminology Criteria for Adverse Events criteria (ver. 4.03), and coded using MedDRA ver. 20.1. Adverse drug reaction (ADR) is a response to a product which is noxious and unintended. Response to a product means that a causal relationship between a product and an AE is at least a reasonable possibility, i.e., the relationship cannot be ruled out. Treatment-emergent adverse event (TEAE) is an undesirable event not present prior to medical treatment, or an already present event that worsens either in intensity or frequency following the treatment.
5. Statistical analysisApproximately 60 patients were planned to be recruited based on feasibility of patient enrollment for a total of 4 years of study duration. Effectiveness and safety analyses sets comprised all eligible patients who received at least one dose of clofarabine.
The primary endpoint of this study was overall remission rate (OR) upon treatment with clofarabine to patients who failed to respond or relapsed after two or more treatment regimens, calculated as the percentage of the sum of number of patients with either CR or CRp after the last clofarabine administration. Secondary endpoints included overall response rate (ORR: percentage of the sum of number of patients with either CR, CRp, or PR after the last clofarabine administration); percentage of patients who underwent HSCT; overall survival (OS: estimated from the date of first dose of clofarabine to death) at 6 months; and safety profile of clofarabine.
Data were summarized using descriptive statistics. Response rates and percentage of patients who received HSCT during the study were presented with 95% confidence intervals (CI). The OS at 6 months was estimated by Kaplan-Meier methods and presented with 95% CI. Log-rank test was used to compare the survival rates between patients with and without HSCT and to estimate the hazard ratio with 95% CI. The OR, ORR, CR, CRp, PR, and refractory rates for HSCT subgroups were also identified. Additionally, OR and ORR were analysed for age, sex, clinical disease status at baseline (first relapse, second relapse, and refractory), time to relapse, the last bone marrow result before study enrollment (CR, CRp, and refractory; the patients were previously diagnosed as CR or CRp but relapsed as R/R ALL and met the eligibility criteria of the study during enrollment), cytogenic subtype (diploid, hypodiploid, hyperdiploid, t(9;22), and others), and immune subtype (B-precursor lineage, T-cell lineage, and mixed) using chi-square test or Fisher exact test. Logistic regression analysis was performed to evaluate predictors for OR and ORR considering variables such as age, sex, the last bone marrow result before study enrollment, cytogenetic subtype and time to relapse. TEAEs were summarized by descriptive statistics. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute, Inc., Cary, NC).
Results1. Patient disposition and baseline characteristicsA total of 60 patients were enrolled and treated with at least one dose of clofarabine; of whom 26 patients (43.3%) completed 6 months of follow-up after the last dose of clofarabine (Fig. 1). Demographics and baseline characteristics are presented in Table 1. The median age of the patients was 12.0 years (range, 2.0 to 26.0 years) and majority were males (37/60; 61.7%). Nearly two-third of the patients (39/60, 65.0%) had B-cell lineage ALL. Baseline assessment revealed that the largest proportion of patients (26/60, 43.3%) had second relapse of disease, followed by refractory disease (21/60, 35.0%) and the first relapse of disease (13/60, 21.7%). Relapses for ≥ 3 times and clinical non-response were categorized as refractory disease according to investigators’ discretion. In addition to the clinical assessment of disease status, the last bone marrow biopsy results before the enrollment were obtained which showed that majority of patients (41/60, 68.3%) had been refractory to the previous treatment.
2. TreatmentFifty-eight patients (96.7%) received clofarabine in combination with other therapies and the remaining patients received clofarabine monotherapy. Overall, mean (standard deviation [SD]) number of clofarabine cycles administered during the study were 1.9±1.0 (median, 2.0; range, 1.0 to 6.0), with mean (SD) dose of 181.9±33.8 mg/m2/cycle (median, 190.4; range, 11.8 to 259.2 mg/m2/cycle), and mean (SD) treatment duration of 5.6±5.8 weeks (median, 5.6; range, 0.1 to 26.7 weeks). The most frequently used other agents in combination therapy were cyclophosphamide and etoposide. The average dose of cyclophosphamide and etoposide was 380 mg/m2/cycle and 150 mg/m2/cycle, respectively.
3. EffectivenessThe OR (CR or CRp) was 45.0% (27/60), while the ORR (CR, CRp, or PR) was 46.7% (28/60) (Table 2). A total of 11 (18.3%), 16 (26.7%), and one (1.7%) patients achieved CR, CRp, and PR, respectively, after the last clofarabine administration. In addition to 27 patients who achieved CR or CRp at the last visit, three patients had achieved CR or CRp during the study period. Among these, 30 patients who achieved remission (CR or CRp) during the course of the study, remission was attained after completing 1 to 3 cycles of clofarabine. The median time to remission and median number of cycles to achieve remission were 5.1 weeks (95% CI, 4.7 to 6.1) and one cycle (range 1 to 3), respectively. The median duration of remission was 16.6 weeks (range, 2.0 to 167.6). Eighteen of the 41 patients (43.9%) with refractory disease prior to enrollment achieved CR or CRp. All patients achieving response had received the combination therapy. Of the two patients who received clofarabine monotherapy, one was refractory to the therapy and data for the other patient was missing.
Of the 16 patients (26.7%) who proceeded to HSCT during the study, six (37.5%) and eight (50.0%) patients achieved CR and CRp, respectively, and the remaining two (12.5%) patients were refractory to the therapy. Of the 44 patients who did not undergo HSCT, five (11.4%), eight (18.2%), and one (2.3%) achieved CR, CRp, or PR, respectively; while 19 patients (43.2%) were refractory to the therapy. The OR in patients receiving HSCT was 87.5% and in those who did not receive HSCT was 29.6% (Table 2). Similarly, ORR was higher in patients receiving HSCT than in those not receiving HSCT (87.5% vs. 31.8%). Baseline and demographic details of patients who received HSCT are presented in Table 1. Despite CR, CRp, or PR, 14 patients did not proceed to HSCT because of toxicity (n=7, of whom 5 had infection), disease relapse (n=3), and follow-up failure (n=3), while one patient was in remission at 6 months. Among the 13 patients who did not proceed to HSCT despite remission due to reason of toxicity, disease relapse, or lost to follow-up, eight patients died, three had relapse of disease and three patients were lost to follow-up.
The OR was higher in proportion of patients with B-cell lineage ALL than with T-cell ALL and mixed phenotype ALL; while the ORR was higher in proportion of patients with mixed phenotype (Table 3). However, these differences in response rates were not statistically significant. Taking into account other baseline characteristics, the OR and ORR were higher in the following subgroups: proportion of patients ≥ 13 years of age; in females; proportion of patients with refractory disease at baseline; proportion of patients having time to relapse ≥ 36 months; proportion of patients with CR on the last bone marrow result before study enrollment; and those with diploid cytogenetic subtype. The OR was significantly higher among patients with time to relapse ≥ 36 months (p=0.030); while the ORR was significantly higher among female patients (p=0.023) and in patients with CR on the last bone marrow result before study enrollment (p=0.022). The difference in both OR and ORR was significantly higher among subgroups by cytogenetic subtypes (p=0.005 and p=0.016, respectively) (Table 3).
Based on p-values of exact logistic regression, the likelihood of higher OR and ORR was significantly influenced by sex, the last bone marrow result before study enrollment, and cytogenetic subtype. Odds of OR and ORR were significantly higher in females versus males (p-value of exact odds ratio; OR: 0.033 and ORR: 0.014) and in patients with CR versus refractory disease on the last bone marrow result before study enrollment (p-value of exact odds ratio; OR: 0.026 and ORR: 0.012) (S1 Table). Diploid versus hyperdiploid cytogenetic subtype and t(9;22) versus hyperdiploid cytogenetic subtype were also associated with higher odds of OR and ORR but these results were not statistically significant.
The 6-month OS rate was 47% (95% CI, 34.0 to 59.0) and the median OS was 23.7 weeks (95% CI, 17.0 to 27.6). Among patients proceeding with HSCT and without HSCT, the median OS was 22.3 months (95% CI, 6.3 to 30.9) and 3.9 months (95% CI, 3.0 to 5.1), respectively. Patients with HSCT had significantly higher survival probability than patients without HSCT (hazard ratio, 0.281; 95% CI, 0.139 to 0.567; p < 0.001) (Fig. 2).
4. SafetyAll patients had at least one TEAE (Table 4). Serious TEAEs were reported in 37 patients (61.7%), and the most common serious TEAEs were febrile neutropenia (28.3%) and sepsis (13.3%) (S2 Table).
Most patients (50/60, 83.3%) had TEAEs assessed as ADRs (Table 4). The most common ADRs were febrile neutropenia (38/60, 63.3%), vomiting (33/60, 55.0%), nausea (32/60, 53.3%), and diarrhea (23/60, 38.3%). About one-third of ADRs (134/383) were grade ≥ 3 in severity (Table 4). The S3 Table summarizes ADRs of varying severity. One-third of the patients (35.0%) had serious TEAEs assessed as ADRs (Table 4).
A total of 24 patients died during the study (Fig. 1). Fourteen patients (23.3%) died due to TEAEs; of these, six (10.0%) died of ADRs (Table 4). The most common ADRs resulting in death were infection and infestation. Five patients died due to fatal infections and infestations including sepsis (n=2, 3.3%), septic shock (n=1, 1.7%), lung infection (n=1, 1.7%) and pneumonia (n=1, 1.7%). Two patients (3.3%) died due to febrile neutropenia (S4 Table).
Thirty-four patients (56.7%) discontinued the study. The most common reason for study discontinuation other than death was disease progression after treatment (n=6) (Fig. 1). Clofarabine was discontinued in six patients (10.0%) due to TEAEs; the most common TEAEs leading to clofarabine discontinuation were febrile neutropenia (5.0%); neutropenia, increased alanine transaminase and aspartate aminotransferase (3.3% each) (S5 Table). Fourteen TEAEs leading to clofarabine discontinuation in three patients (5.0%) were assessed as ADRs (S4 Table).
DiscussionClofarabine monotherapy or combination therapy showed OR of 45.0% and ORR of 46.7%, irrespective of the immunophenotype and despite a high proportion of patients (68.3%) refractory to the previous treatment.
In comparison to previous phase 2 study conducted in 25 patients (age, 1 to 21 years) with R/R ALL who received 1–3 cycles of clofarabine, cyclophosphamide, and etoposide combination therapy, the OR in the present study was similar (45% vs. 44%) and the ORR was lower (46.7% vs. 56%) [19]. In another phase 2 study conducted in 25 patients (age, 4 to 21 years) with R/R ALL, who received a single course of clofarabine, cyclophosphamide, and etoposide combination therapy daily for 5 days, the OR was 56% [20]. In a third phase 2 study, 61 patients (age, 1 to 20 years) with R/R ALL received clofarabine monotherapy daily for 5 days, every 2–6 weeks; the OR and ORR reported were 20% and 30%, respectively [18].
In this study, logistic regression analysis indicated that the likelihood of higher OR and ORR was more among patients with CR than those with refractory disease on the last bone marrow result before study enrollment and in female patients compared with male patients. An earlier study has reported lower survival rate after relapse in males with ALL despite more intensive frontline therapy [21]. However, there are no clear evidences to show that treatment with clofarabine results in higher OR and ORR in a particular sex. Similarly, no difference in clofarabine pharmacokinetics was observed between male and female patients in an earlier pharmacokinetic study [22]. Therefore, difference in response rates between male and female patients observed in this study cannot be attributed to sex but may be due to unidentified differences in individual characteristics of this subgroup. This study also recommends further assessment of other subgroups having lower response with clofarabine treatment.
In Chopin study (a retrospective analysis), the survival of patients with HSCT was significantly higher compared with those without HSCT (35.2% vs. 0%) [15]. These results are similar to the present prospective study. It is a known fact that patients who do not achieve CR but undergo HSCT have shown dismal prognosis [10]. This elucidates the need for novel therapy to induce durable CR response and provide bridge to HSCT in R/R pediatric ALL patients. In a previous clofarabine monotherapy study, among nine patients proceeding with HSCT, two patients each achieved CR and CRp and one patient achieved PR; whereas, four patients who did not receive transplantation maintained a durable CR or CRp [18]. In the present study, 43.9% patients with refractory disease showed CR or CRp. Clofarabine induced CR or CRp even in patients without HSCT. It may have the potential to prolong the time to achieve HSCT in pediatric patients with R/R ALL.
Five patients in present study did not proceed to HSCT, despite CR, CRp, or PR, because of infection. TEAE Infection rate of the total patients (70%) is consistent with the previous studies [19,20]. The HSCT could not be implemented due to an infection, indicating that clofarabine monotherapy or combination therapy may have resulted in severe immune and myelosuppression in these patients receiving prior treatments. The safety profile of clofarabine in this study was consistent with that observed in the earlier studies and is as expected in the previously treated population [18,19].
A total of 24 deaths were reported during the 6-month follow-up period; 14 patients died due to grade 5 TEAEs, of whom, six patients died due to grade 5 ADRs. The cause of death other than TEAEs for the remaining 10 patients was not reported. Infection was the most reported TEAE (11/14 patients) and ADR (5/6 patients) leading to death. Of the six deaths due to ADRs, one patient had reported both, infection as well as febrile neutropenia. During chemotherapy, patients are susceptible to infection that can lead to sepsis, which can be life-threatening due to weakened immune system and myelosuppression [23,24]. Infection control (especially in the event of neutropenia or lymphopenia during clofarabine treatment) must be prioritized by thorough monitoring of infection prophylaxis and early recognition of infection sign for safety management [25].
Blinatumomab, a bispecific monoclonal antibody, is emerging as an alternative for CD19-positive ALL and has been approved for use in patients with R/R B-cell precursor ALL [26]. Despite being an effective treatment option for R/R ALL, some patients experienced relapse post-treatment with blinatumomab. After the failure of blinatumomab, the treatment of these patients remains an unmet medical need [27]. U.S. Food and Drug Administration approved chimeric antigen receptor T-cell (CAR-T) therapy for pediatric ALL and it is an important new treatment option that has shown durable response [28–30]. However, CAR-T therapy is yet to be tested for long-term response compared with HSCT and has some limitations such as severe AEs and resistance that obstruct its implementation in clinical practice [31,32]. In the age of CAR-T, there are still unmet medical needs. Clofarabine can become an important treatment option for patients who have CD19-negative ALL, and for some reason are unable to receive blinatumomab or CAR-T therapy, or have failed to respond to new novel therapies.
As this was an observational study, the information whether the patient received HSCT before enrollment in this study was not captured. Minimal residual disease (MRD) is an important prognostic factor, both, before and after transplantation; a negative MRD before transplantation is strongly associated with a good prognosis [33,34]. An additional analysis of treatment-induced MRD in this study would have facilitated better prediction of outcome of HSCT. As most of the patients received combination therapy in this study, it was difficult to compare monotherapy versus combination therapy and can be considered as a limitation. As the 6-month OS rate is not informative in children compared with adults, the patients were followed up for longer period (3 years from the initiation of the study) to know their survival status. However, the 3-year OS rates were found to be below 10%. Most of the patients who had not attained CR or CRp did not receive HSCT; hence, there is a need of a vigilant interpretation of the differences in OR and ORR between patients with and without HSCT. This observation implies that the OR and ORR comparison between patients receiving HSCT and not receiving HSCT may not be meaningful. Nevertheless, OS curves showed the real-world longer-term survival of patients with HSCT compared to patients without HSCT.
To conclude, this study indicates a novel alternative treatment for pediatric R/R ALL patients who have limited treatment options and low long-term survival rates. The study showed acceptable OR and ORR as well as expected safety profile with clofarabine in Korean pediatric patients with R/R ALL; these study findings are in line with the results reported in the overall population treated with clofarabine. In addition, the observed infections and HSCT transition rates imply that treatment of R/R ALL remains challenging. Although remission was accomplished in some patients, it eventually did not improve survival outcomes. Long-term survival outcome was still dismal in this study.
To improve the performance of HSCT, further studies are required to develop MRD directed therapy, and reduce toxicities. Nevertheless, clofarabine showed meaningful results and is expected to broaden treatment strategies available to R/R Korean pediatric ALL patients. Clofarabine could be an option for those countries struggling for treatment options due to lack of reimbursement for an unauthorized drug by an insurance company. Findings from this study may facilitate decision making with regard to insurance coverage of pediatric patients with ALL in Korea who require clofarabine as a treatment option.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (https://www.e-crt.org).
NotesEthical Statement The study protocol was approved by the local institutional review boards (IRB of Seoul National University: H-1401-140-552). Parents or legal representative of patients provided written informed consent for participation in the study. In addition, patients ≥ 7 and < 12 years of age provided verbal consent; while, patients ≥ 12 years of age provided written consent. The study was conducted in accordance with the basic principles of the Declaration of Helsinki. The study complied with the guidelines for Good Epidemiology Practice and national laws and regulations of Korea. Author Contributions Conceived and designed the analysis: Choi JY, Hong CR, Hong KT, Kang HJ, Kim S, Lee JW, Jang PS, Chung NG, Cho B, Kim H, Koh KN, Im HJ, Seo JJ, Hahn SM, Han JW, Lyu CJ, Yang EJ, Lim YT, Yoo KH, Koo HH, Kook H, Jeon IS, Cho H, Shin HY. Collected the data: Choi JY, Hong CR, Hong KT, Kang HJ, Kim S, Lee JW, Jang PS, Chung NG, Cho B, Kim H, Koh KN, Im HJ, Seo JJ, Hahn SM, Han JW, Lyu CJ, Yang EJ, Lim YT, Yoo KH, Koo HH, Kook H, Jeon IS, Cho H, Shin HY. Performed the analysis: Hong CR, Hong KT, Kang HJ, Kim S, Lee JW, Jang PS, Chung NG, Cho B, Kim H, Koh KN, Im HJ, Seo JJ, Hahn SM, Han JW, Lyu CJ, Yang EJ, Lim YT, Yoo KH, Koo HH, Kook H, Jeon IS, Cho H, Shin HY. Wrote the paper: Cho H, Shin HY. Reviewed the manuscript: Choi JY, Hong CR, Hong KT, Kang HJ, Kim S, Lee JW, Jang PS, Chung NG, Cho B, Kim H, Koh KN, Im HJ, Seo JJ, Hahn SM, Han JW, Lyu CJ, Yang EJ, Lim YT, Yoo KH, Koo HH, Kook H, Jeon IS, Cho H, Shin HY. AcknowledgmentsThe study was funded by Sanofi Aventis Korea Ltd. The authors would like to thank the study participants, their family, and caregivers who were involved in this study. SiYeon Kil was involved in statistical analyses. Writing support was provided by Rukhsar Wasta with editorial assistance of Sonal More (both from Tata Consultancy Services India Ltd.) in the preparation of this publication and paid for by Sanofi. Editorial support was also provided by Anahita Gouri and Rohan Mitra of Sanofi, India. The authors, individually and collectively, are responsible for all content and editorial decisions and received no payment from Sanofi directly or indirectly (through a third party) related to the development/presentation of this publication.
Fig. 2Survival status with clofarabine use. Overall survival (A) and survival by HSCT (B). CI, confidence interval; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation. 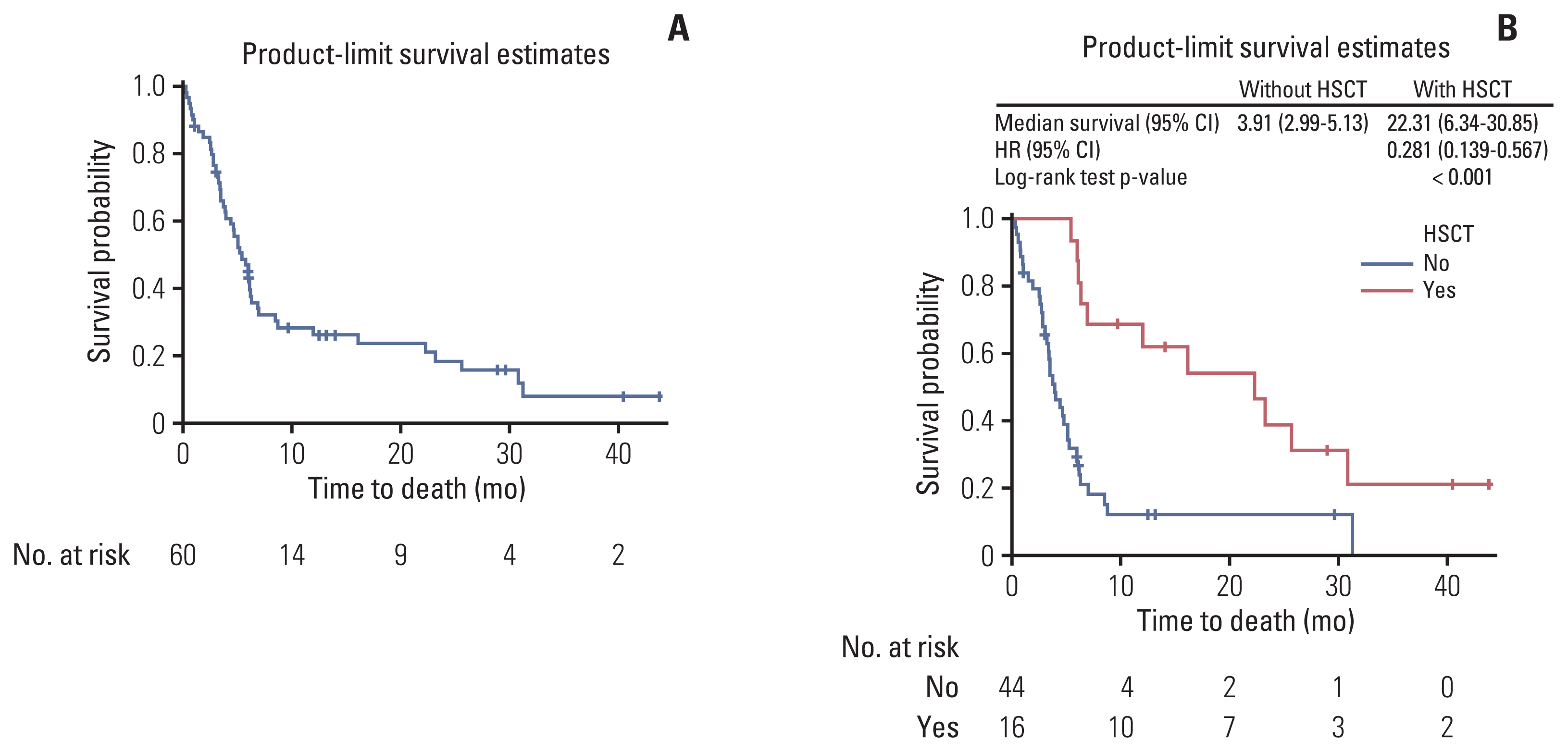
Table 1Demographics and baseline characteristics
Values are presented as number (%) unless otherwise indicated. Denominator of percentage is the number of patients in each category. Age (yr)=(informed consent date [yyyy])−(birth date [yyyy])+1, if (informed consent date [mm/dd]) < ([birth date (mm/dd)] or (informed consent date [yyyy])−(birth date [yyyy]), if (informed consent date [mm/dd]) ≥ (birth date [mm/dd]). Time from initial diagnosis=(informed consent date [yyyy])−(ALL diagnosis date [yyyy])+1. ALL, acute lymphoblastic leukemia; CR, complete remission; CRp, CR without platelet recovery; HSCT, hematopoietic stem cell transplantation; Max, maximum; Min, minimum; NA, not available. Table 2Overall remission rate and overall response rate with clofarabine use, with and without HSCT
Values are presented as number (%) unless otherwise indicated. Denominator of percentage is the number of patients in each group. CI, confidence interval; CR, complete remission; CRp, CR without platelet recovery; HSCT, hematopoietic stem cell transplantation; PR, partial remission. a) Refractory represents ≥ 3 relapse and/or clinically non-responsive disease according to investigators’ discretion, b) Overall remission rate (%)=(CR after the last clofarabine administration+CRp after the last clofarabine administration)/(total number of treated patients)×100, Table 3Comparison of overall remission rate and overall response rate by baseline characteristics
Values are presented as number (%). Denominator of percentage is the number of patients in each group. CR, complete remission; CRp, CR without platelet recovery; PR, partial remission. Table 4Overall summary of TEAEs and ADRs with clofarabine use (n=60)
Denominator of percentage is the number of patients in each group. Grade 1, mild; grade 2, moderate; grade 3, severe or medically significant but not immediately life-threatening; grade 4, life-threatening consequences; grade 5, death related to AE. ADRs, adverse drug reactions; AE, adverse events; TEAEs, treatment-emergent adverse events. References1. Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr Int. 2018;60:4–12.
2. Boer JM, den Boer ML. BCR-ABL1-like acute lymphoblastic leukaemia: from bench to bedside. Eur J Cancer. 2017;82:203–18.
3. Stary J, Hrusak O. Recent advances in the management of pediatric acute lymphoblastic leukemia [version 1; peer review: 2 approved]. F1000Res. 2016;5:2635.
4. Park HJ, Moon EK, Yoon JY, Oh CM, Jung KW, Park BK, et al. Incidence and survival of childhood cancer in Korea. Cancer Res Treat. 2016;48:869–82.
5. Kim H. Recent advances in the treatment of pediatric acute leukemia. J Korean Med Assoc. 2016;59:690–7.
6. Pui CH. Recent research advances in childhood acute lymphoblastic leukemia. J Formos Med Assoc. 2010;109:777–87.
7. Ju HY, Hong CR, Shin HY. Advancements in the treatment of pediatric acute leukemia and brain tumor: continuous efforts for 100% cure. Korean J Pediatr. 2014;57:434–9.
8. Ceppi F, Duval M, Leclerc JM, Laverdiere C, Delva YL, Cellot S, et al. Improvement of the outcome of relapsed or refractory acute lymphoblastic leukemia in children using a risk-based treatment strategy. PLoS One. 2016;11:e0160310.
9. Fuster JL. Current approach to relapsed acute lymphoblastic leukemia in children. World J Hematol. 2014;3:49–70.
10. Duval M, Klein JP, He W, Cahn JY, Cairo M, Camitta BM, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–8.
11. Inagaki J, Fukano R, Noguchi M, Kurauchi K, Tanioka S, Okamura J. Hematopoietic stem cell transplantation following unsuccessful salvage treatment for relapsed acute lymphoblastic leukemia in children. Pediatr Blood Cancer. 2015;62:674–9.
12. Jeha S, Kantarjian H. Clofarabine for the treatment of acute lymphoblastic leukemia. Expert Rev Anticancer Ther. 2007;7:113–8.
13. Raetz EA, Bhatla T. Where do we stand in the treatment of relapsed acute lymphoblastic leukemia? Hematology Am Soc Hematol Educ Program. 2012;2012:129–36.
14. Pession A, Masetti R, Kleinschmidt K, Martoni A. Use of clofarabine for acute childhood leukemia. Biologics. 2010;4:111–8.
15. Yoo KH, Chung NG, Cho B, Kang HJ, Shin HY, Im HJ, et al. A multicenter retrospective analysis on the treatment pattern and outcome in relapsed/refractory childhood acute lymphoblastic leukemia. Clin Pediatr Hematol Oncol. 2017;24:101–6.
16. Yoo SL, Kim DJ, Lee SM, Kang WG, Kim SY, Lee JH, et al. Improving patient access to new drugs in South Korea: evaluation of the national drug formulary system. Int J Environ Res Public Health. 2019;16:e288.
17. Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) Co-operative Group. Br J Haematol. 1976;33:451–8.
18. Jeha S, Gaynon PS, Razzouk BI, Franklin J, Kadota R, Shen V, et al. Phase II study of clofarabine in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. J Clin Oncol. 2006;24:1917–23.
19. Hijiya N, Thomson B, Isakoff MS, Silverman LB, Steinherz PG, Borowitz MJ, et al. Phase 2 trial of clofarabine in combination with etoposide and cyclophosphamide in pediatric patients with refractory or relapsed acute lymphoblastic leukemia. Blood. 2011;118:6043–9.
20. Locatelli F, Testi AM, Bernardo ME, Rizzari C, Bertaina A, Merli P, et al. Clofarabine, cyclophosphamide and etoposide as single-course re-induction therapy for children with refractory/multiple relapsed acute lymphoblastic leukaemia. Br J Haematol. 2009;147:371–8.
21. Nguyen K, Devidas M, Cheng SC, La M, Raetz EA, Carroll WL, et al. Factors influencing survival after relapse from acute lymphoblastic leukemia: a Children’s Oncology Group study. Leukemia. 2008;22:2142–50.
22. Bonate PL, Cunningham CC, Gaynon P, Jeha S, Kadota R, Lam GN, et al. Population pharmacokinetics of clofarabine and its metabolite 6-ketoclofarabine in adult and pediatric patients with cancer. Cancer Chemother Pharmacol. 2011;67:875–90.
23. Barreto JN, McCullough KB, Ice LL, Smith JA. Antineoplastic agents and the associated myelosuppressive effects: a review. J Pharm Pract. 2014;27:440–6.
24. Nordvig J, Aagaard T, Daugaard G, Brown P, Sengelov H, Lundgren J, et al. Febrile neutropenia and long-term risk of infection among patients treated with chemotherapy for malignant diseases. Open Forum Infect Dis. 2018;5:ofy255.
25. Food and Drug AdministrationHighlights of prescribing information: Clolar (clofarabine) [Internet]. Silver Spring MD: Food and Drug Administration; 2015. [cited 2021 Jan 2]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/021673s024lbl.pdf
26. von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, et al. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol. 2016;34:4381–9.
27. Jabbour E, Dull J, Yilmaz M, Khoury JD, Ravandi F, Jain N, et al. Outcome of patients with relapsed/refractory acute lymphoblastic leukemia after blinatumomab failure: no change in the level of CD19 expression. Am J Hematol. 2018;93:371–4.
28. Hucks G, Rheingold SR. The journey to CAR T cell therapy: the pediatric and young adult experience with relapsed or refractory B-ALL. Blood Cancer J. 2019;9:10.
29. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–48.
30. Kenderian SS, Porter DL, Gill S. Chimeric antigen receptor T cells and hematopoietic cell transplantation: how not to put the CART before the horse. Biol Blood Marrow Transplant. 2017;23:235–46.
31. Forsberg MH, Das A, Saha K, Capitini CM. The potential of CAR T therapy for relapsed or refractory pediatric and young adult B-cell ALL. Ther Clin Risk Manag. 2018;14:1573–84.
32. Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16:372–85.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||