AbstractPurposeThis study aimed to analyze whether patients with lung cancer have a higher susceptibility of coronavirus disease 2019 (COVID-19), severe presentation, and higher mortality than those without lung cancer.
Materials and MethodsA nationwide cohort of confirmed COVID-19 (n=8,070) between January 1, 2020, and May 30, 2020, and a 1:15 age-, sex-, and residence-matched cohort (n=121,050) were constructed. A nested case-control study was performed to compare the proportion of patients with lung cancer between the COVID-19 cohort and the matched cohort.
ResultsThe proportion of patients with lung cancer was significantly higher in the COVID-19 cohort (0.5% [37/8,070]) than in the matched cohort (0.3% [325/121,050]) (p=0.002). The adjusted odds ratio [OR] of having lung cancer was significantly higher in the COVID-19 cohort than in the matched cohort (adjusted OR, 1.51; 95% confidence interval [CI], 1.05 to 2.10). Among patients in the COVID-19 cohort, compared to patients without lung cancer, those with lung cancer were more likely to have severe COVID-19 (54.1% vs. 13.2%, p < 0.001), including mortality (18.9% vs. 2.8%, p < 0.001). The adjusted OR for the occurrence of severe COVID-19 in patients with lung cancer relative to those without lung cancer was 2.24 (95% CI, 1.08 to 4.74).
IntroductionThe infection caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), named coronavirus disease 2019 (COVID-19) by the World Health Organization (WHO), has spread rapidly worldwide since it was first reported in Wuhan, China, at the end of December 2019 [1,2]. The new virus has affected more than 200 countries and territories to date and continues to spread further [2,3].
Patients with cancer, especially those with lung cancer, are suggested to be vulnerable to COVID-19. Previous studies have shown that the rate of COVID-19 in cancer patients is higher than that in the general population [4–7], and lung cancer is the most common cancer subtype. However, these studies included patients with all type of cancers and did not control for important demographic characteristics (e.g., age, sex, and socioeconomic status) [6] and pulmonary comorbidities (e.g., chronic obstructive pulmonary disease [COPD] and asthma) [8,9], which have high susceptibility to COVID-19.
According to the available data, 5%–20% of COVID-19 patients have severe disease [1,3,10,11], which is mainly characterized by acute respiratory distress syndrome. Patients with lung cancer are suggested to be at a higher risk of this severe form of COVID-19 [6,7,12,13]. Recent studies have shown that the mortality rate of patients with lung cancer is higher than that of the general population when infected with COVID-19 [12,13]. However, as these studies were conducted in a single center, they did not directly compare the severity of COVID-19 and mortality between patients with lung cancer and those without lung cancer or the general population. They also did not consider clinical characteristics such as age, sex, and common pulmonary comorbidities in patients with lung cancer.
Therefore, this study aimed to investigate whether lung cancer patients are more susceptible to COVID-19 than patients without lung cancer. Furthermore, we evaluated the effect of lung cancer on the development of severe COVID-19.
Materials and Methods1. Study populationThe Korean government provided researchers with anonymized national patient data for the evaluation of COVID-19 [14]. This large cohort provides Korea National Health Insurance records claimed between January 1, 2015, and May 30, 2020, and consists of the three sub-cohorts: (1) the COVID-19 cohort comprised of patients who had a confirmed diagnosis of COVID-19 after January 2020 (n=8,070), (2) the inspection control group that included patients whose COVID-19–related code (B342, B972, U071, and U072) has been requested after January 2020, however, excluded those who had confirmed COVID-19 (n=222,257), and (3) the matched cohort that included a 15-fold population matched with the COVID-19 cohort by age, sex, and residential area (n=121,050). Of the data provided, we used data from (1) the COVID-19 cohort and (3) the matched cohort (Fig. 1).
The data in this cohort were combined with the claims-based data from the National Health Insurance Service (NHIS) between January 1, 2015, and May 30, 2020, and we extracted information on age, sex, and region of residence from the insurance eligibility data. Therefore, the datasets analyzed in this study include records on personal data, healthcare records of inpatients and outpatients from the past 5 years (including healthcare visits, prescriptions, diagnoses, and procedures), pharmaceutical visits, COVID-19–related outcomes, and death records.
2. DefinitionLaboratory confirmation of SARS-CoV-2 infection was defined as a positive result on a real-time reverse-transcriptase polymerase chain reaction assay of nasal or pharyngeal swabs, in accordance with the WHO guidelines [15]. Lung cancer was defined as ≥ 2 claims under the International Classification of Diseases, 10th revision (ICD-10) code C34. We defined comorbidities as comorbidities with ≥ 2 claims under ICD-10 codes as a major diagnosis during the study period (from January 1, 2015, to May 30, 2020), as follows: angina pectoris (I20), myocardial infarction (I21, I22, or I25.2), asthma (J45-J46), COPD (J42-J44 except for J43.0 [unilateral emphysema]), cerebrovascular disease (G45-G46, I60-I69, or H34.0), diabetes mellitus (E10-E14), hypertension (I10-I15), heart failure (I43, I50, I09.9, I11.0, I25.5, I13.0, I13.2, I42.0, I42.5-I42.9, or P29.0), malignancy other than lung cancer (C00-C97, except for C34), and rheumatologic disease (M05, M06, M31.5, M32, M33, M34, M35.1, M35.3, or M36.0) [16]. Severe COVID-19 was defined as cases with the need for oxygen therapy, prompt care in the intensive care unit (ICU), and mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) treatment in addition to patients who died after a confirmed COVID-19 diagnosis [5,6,17].
3. Main outcomes and measuresThe primary objective of this study was to evaluate whether patients with lung cancer are more vulerable to COVID-19 compared to those without lung cancer. The secondary objective was to evaluate the effect of lung cancer on the development of severe COVID-19.
4. Statistical analysisCategorical variables are presented as number (%) and compared using chi-square test or Fisher exact test, as appropriate.
To evaluate the effect of lung cancer on COVID-19 susceptibility, the odds ratio (OR) for lung cancer in the COVID-19 cohort relative to the age- and sex-matched cohort was evaluated using univariable and multivariable analyses. Model 1 was adjusted for type of insurance. Model 2 was additionally adjusted for asthma and COPD.
To evaluate the effect of lung cancer on the occurrence of severe COVID-19, we compared the odds for severe COVID-19 in patients with lung cancer versus those in patients without lung cancer using the COVID-19 cohort. Model 1 was adjusted for age and sex. Model 2 was additionally adjusted for asthma and COPD. All statistical analyses were performed using SAS ver. 9.4 (SAS Institute Inc., Cary, NC).
Results1. Baseline characteristicsThe COVID-19 and matched cohorts were well balanced in terms of age and sex. The proportion of patients who received medical aid was higher in the COVID-19 cohort than in the matched cohort (7.3% vs. 3.7%, p < 0.001). Regarding pulmonary comorbidities, the rates of lung cancer (0.7% vs. 0.3%, p < 0.001), asthma (19.6% vs. 17.7%, p < 0.001), and COPD (9.4% vs. 7.4%, p < 0.001) were higher in the COVID-19 cohort than in the matched cohort. The rates of extra-pulmonary comorbidities were also significantly higher in the COVID-19 cohort than in the matched cohort, except for malignancy other than lung cancer (Table 1).
2. Risk of COVID-19 occurrence in patients with lung cancerThe proportion of patients with lung cancer was significantly higher in the COVID-19 cohort than in the matched cohort (0.5% vs. 0.3%, p=0.002) (Table 1). As shown in Table 2, the odds for lung cancer in the COVID-19 cohort was 1.71-fold higher than that in the matched cohort (unadjusted OR, 1.71; 95% CI, 1.20 to 2.37). The significant difference persisted after further adjustment for potential confounding factors in model 1 (adjusted OR, 1.69; 95% CI, 1.18 to 2.35) and model 2 (adjusted OR, 1.51; 95% CI, 1.05 to 2.10).
3. Clinical characteristics of COVID-19 patients according to the presence of lung cancerOf the 8,070 COVID-19 patients, 37 (0.5%) had lung cancer. COVID-19 patients with lung cancer were characterized by significantly older age than those without lung cancer (p < 0.001). There were no significant differences in sex and type of insurance between COVID-19 patients with lung cancer and those without lung cancer. COVID-19 patients with lung cancer showed significantly higher comorbid conditions of asthma (37.8% vs. 19.5%, p=0.005) and COPD (48.7% vs. 9.3%, p < 0.001) than those without lung cancer. COVID-19 patients with lung cancer had more extra-pulmonary comorbidities than those without lung cancer, such as hypertension, diabetes mellitus, cerebrovascular diseases, angina pectoris, myocardial infarction, heart failure, and malignancy other than lung cancer (Table 3).
4. Risk of severe COVID-19 in patients with lung cancerCOVID-19 patients with lung cancer showed more severe clinical course than those without lung cancer, requiring the following treatment options: oxygen therapy (cases receiving mechanical ventilation or ECMO and mortality cases were not included) (48.7% vs. 12.4%, p < 0.001), ICU admission (16.2% vs. 2.9%, p < 0.001), and mechanical ventilation (cases receiving ECMO and mortality cases were not included) (10.8% vs. 1.9%, p < 0.001). COVID-19 patients with lung cancer also demonstrated significantly higher mortality than those without lung cancer (18.9% vs. 2.8%, p < 0.001) (Table 4).
Table 5 shows the effect of lung cancer on severe COVID-19 among patients in the COVID-19 cohort. In univariable analysis, patients with lung cancer were 7.75 times more likely to have severe COVID-19 (unadjusted OR, 7.75; 95% CI, 4.05 to 15.01) than those without lung cancer. The significant difference persisted after further adjustment for potential confounding factors in model 1 (adjusted OR, 2.69; 95% CI, 1.31 to 5.61) and model 2 (adjusted OR, 2.24; 95% CI, 1.08 to 4.74).
DiscussionIn this nested case-control study using a nationwide COVID-19 cohort and an age, sex, and region-matched cohort, the odds for lung cancer in the COVID-19 cohort were 1.5-fold higher compared to those in the matched cohort, even after adjustment for potential confounding factors including socioeconomic status and comorbid pulmonary diseases. In addition, this study revealed that COVID-19 patients with lung cancer had more severe clinical course, including higher mortality, than COVID-19 patients without lung cancer.
Although the effect of pulmonary comorbidities such as asthma and COPD on the susceptibility of COVID-19 was relatively well evaluated [8,18], there was very limited information on whether patients with lung cancer have a higher risk of COVID-19 than those without lung cancer. Yu et al. [7] suggested that the risk of COVID-19 in patients with cancer, especially those with lung cancer, is higher than that in the general population by comparing the rate of COVID-19 among cancer patients with that in the general population. However, this was a single-center study, which may limit the study findings. In line with our results, Liang et al. [6] showed that patients with cancer—especially lung cancer—are at a higher risk of COVID-19 than the general population using a nationwide analysis in China. However, this study did not consider important biases (e.g., age, sex, and socioeconomic status) when they compared the incidence of COVID-19 between the cancer patient cohort and the general population. Additionally, both these aforementioned studies evaluated all type of cancers. The strength of our study lies in the fact that we evaluated only patients with lung cancer and controlled for important biases using the nationwide COVID-19 cohort and the matched cohort. We also controlled for socioeconomic status comorbidities using adjustment and stratification.
In this study, patients with lung cancer were found to be not only more susceptible to COVID-19 but also likely to experience more severe disease and have higher mortality than those without lung cancer. In line with our finding, recent studies reported that COVID-19 imposes a high burden of severity on and increased mortality in patients with lung cancer [4,5,12,13]. However, these studies did not compare the seve-rity of COVID-19 and mortality between patients with lung cancer and those without lung cancer or the general population. As a simple comparison without considering bias can exaggerate the results, controlling biases is important to estimate the real effect of lung cancer on the progression of severe COVID-19. We showed that a very high unadjusted OR sharply reduced after adjusting for important biases. After controlling for these variables, the risk of severe COVID-19 was about 2-fold higher in patients with lung cancer than in those without lung cancer.
There are also limitations to this study. First, since the national insurance claims data were used, information on functional status was not available in this study. In addition to limited clinical information that may affect the survival of patients with lung cancer, this study was conducted in a single country, which may limit its generalizability. Second, the number of lung cancer patients was relatively small, which may be associated with a relatively large range of CIs and insignificant results in subgroup analysis. Third, our dataset lacked specific information on lung cancer, such as pathologic types, current cancer stage, and prior treatments for lung cancer because the NHIS database did not provide this information. Fourth, all COVID-19 patients were admitted and isolated in hospitals in Korea, and we could not compare the rate of hospitalization between lung cancer patients and those without lung cancer. Thus, we did not include hospital admission as an index of disease severity. Fifth, this study could not provide the specific causes of death in COVID-19 patients with lung cancer, since the information was not available in the NHIS COVID-19 database.
This is the first study to assess the effect of lung cancer on patient’s susceptibility to COVID-19 and severe presentation, including mortality. Patients with lung cancer may be more vulnerable to COVID-19. In addition, COVID-19 patients with lung cancer may show more severe clinical course than those without lung cancer. Hence, pulmonologists and thoracic oncologists should pay more attention to patients with lung cancer during this COVID-19 pandemic era.
NotesEthical Statement The Institutional Review Board of our institution approved this study (application No. HYUH 2020-06-029). Since the NHIS database was constructed after anonymization, the need for informed consent from the participants was waived. Author Contributions Conceived and designed the analysis: Yang B, Choi H, Chung SJ, Park DW, Park TS, Kim TH, Sim YS, Yoon HJ, Sohn JW, Lee H, Kim SH. Collected the data: Yang B, Choi H, Lee H, Kim SH. Contributed data or analysis tools: Yang B, Choi H, Lee SK, Lee H, Kim SH. Performed the analysis: Yang B, Choi H, Lee SK, Lee H, Kim SH. Wrote the paper: Yang B, Choi H, Lee SK, Chung SJ, Yeo Y, Shin YM, Park DW, Park TS, Moon JY, Kim TH, Lee H, Kim SH. AcknowledgmentsThis research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HC19C0318).
Fig. 1Flow chart of the study population. COVID-19, coronavirus disease 2019; NHIS, National Health Insurance Service. 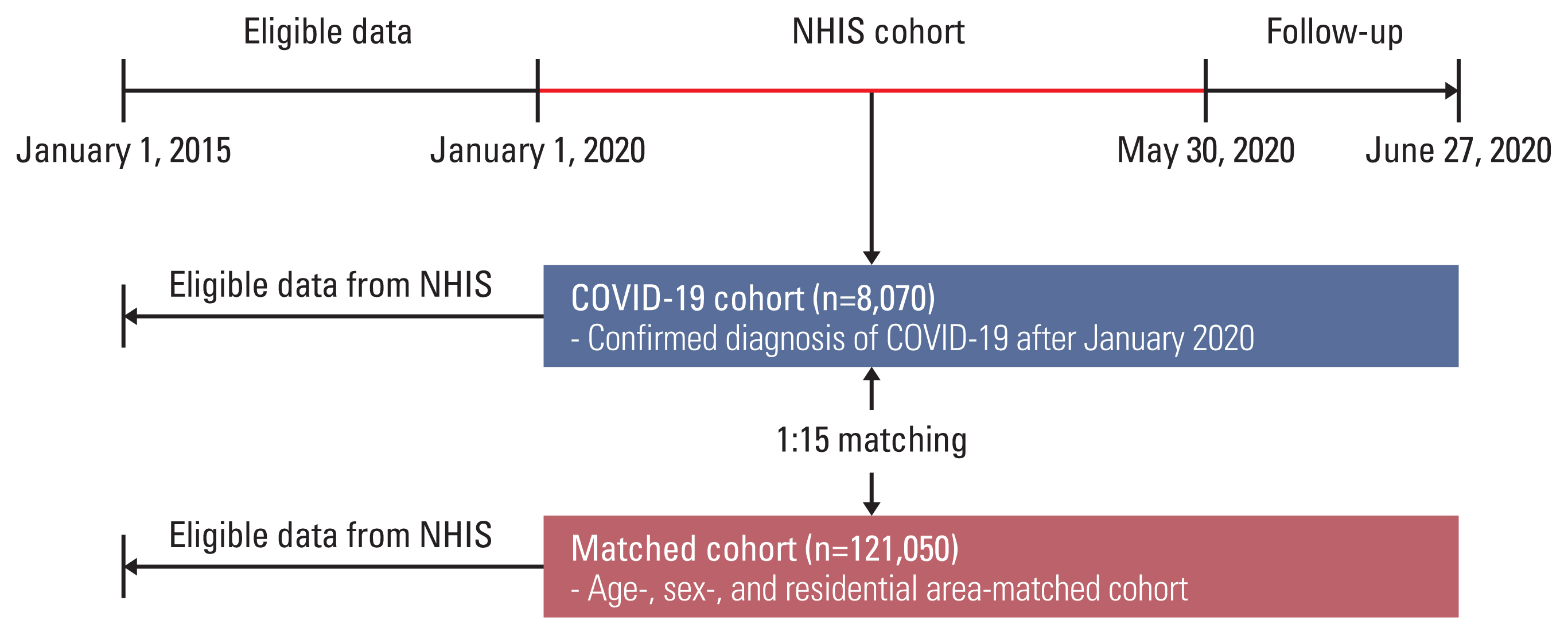
Table 1Baseline characteristics Table 2Adjusted odds ratios (95% confidence intervals) for lung cancer in the COVID-19 cohort and the matched cohort Table 3Clinical characteristics of COVID-19 patients according to the presence or absence of lung cancer Table 4Comparison of most severe clinical course of COVID-19 disease according to the presence or absence of lung cancer Values are presented as number (%). Patients receiving oxygen therapy did not include cases receiving mechanical ventilation treatment or ECMO and mortality cases; those receiving mechanical ventilation treatment did not include cases receiving ECMO and mortality cases; those receiving ECMO did not include mortality cases. COVID-19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; ICU, intensive care unit. References1. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20.
2. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–13.
3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42.
4. Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–6.
5. Garassino MC, Whisenant JG, Huang LC, Trama A, Torri V, Agustoni F, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol. 2020;21:914–22.
6. Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–7.
7. Yu J, Ouyang W, Chua ML, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol. 2020;6:1108–10.
8. Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15:e0233147.
9. Yang JM, Koh HY, Moon SY, Yoo IK, Ha EK, You S, et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146:790–8.
10. Grasselli G, Pesenti A, Cecconi M. Critical care utilization for the COVID-19 outbreak in Lombardy, Italy: early experience and forecast during an emergency response. JAMA. 2020;323:1545–6.
11. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81.
12. Rogado J, Pangua C, Serrano-Montero G, Obispo B, Marino AM, Perez-Perez M, et al. Covid-19 and lung cancer: a greater fatality rate? Lung Cancer. 2020;146:19–22.
13. Luo J, Rizvi H, Preeshagul IR, Egger JV, Hoyos D, Bandlamudi C, et al. COVID-19 in patients with lung cancer. Ann Oncol. 2020;31:1386–96.
14. National Health Insurance Sharing Service [Internet]. Wonju: National Health Insurance Sharing Service; 2019. [cited 2020 Dec 26]. Available from: https://nhiss.nhis.or.kr/bd/ay/bday-a001iv.do
15. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, et al. Renin-angiotensin-aldosterone system inhibitors and risk of COVID-19. N Engl J Med. 2020;382:2441–8.
16. Choi H, Yang B, Nam H, Kyoung DS, Sim YS, Park HY, et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J. 2019;54:1900194.
|
|
||||||||||||||||||||||||||||||||||||||||||||