AbstractPurposeThe purpose of this study was to investigate the effect of 21-gene recurrence score (RS) on predicting prognosis and chemotherapy decision in node micrometastases (N1mi) breast invasive ductal carcinoma (IDC).
MethodsPatients with stage T1-2N1mi and estrogen receptor-positive IDC diagnosed between 2004 and 2015 were included. The associations of 21-gene RS with breast cancer-specific survival (BCSS), chemotherapy decision, and benefit of chemotherapy were analyzed.
ResultsWe identified 4,758 patients including 1,403 patients (29.5%) treated with adjuvant chemotherapy. In the traditional RS cutoffs, 2,831 (59.5%), 1,634 (34.3%), and 293 (6.2%) patients were in the low-, intermediate-, and high-risk RS groups, respectively. In 3,853 patients with human epidermal growth factor receptor-2 (HER2) status available, most patients were HER2-negative disease (98.3%). A higher RS was independently related to chemotherapy receipt, and 14.0%, 47.7%, and 77.8% of patients in the low-, intermediate-, and high-risk RS groups received chemotherapy, respectively. The multivariate analysis indicated that a higher RS was related to worse BCSS (p < 0.001). The 5-year BCSS rates were 99.3%, 97.4%, and 91.9% in patients with low-, intermediate-, and high-risk RS groups, respectively (p < 0.001). However, chemotherapy receipt did not correlate with better BCSS in low-, intermediate-, or high-risk RS groups. There were similar trends using Trial Assigning Individualized Options for Treatment RS cutoffs.
IntroductionWith advances in diagnostics, histopathology, and molecular analysis of breast cancer, an increasing number of patients are being diagnosed with node micrometastatic disease (≤ 2 mm axillary node metastasis, N1mi) [1-3], accounting for approximately 15% of all node-positive patients [4]. There is dispute about the clinical value of N1mi disease in breast cancer. Several early studies showed similar outcomes between node-negative (N0) and N1mi breast cancer [5-7]. The treatment algorithms in N1mi disease are regarded as equal to N0 disease in the current National Comprehensive Cancer Network (NCCN) guidelines [8]. However, in more recent studies, patients with N1mi disease had worse prognosis compared to those with N0 disease [9,10]. There is a paucity of prospective studies to address the effectiveness of chemotherapy in N1mi patients. Gene expression profiling can provide better risk stratification than traditional clinicopathologic characteristics and can more accurately predict the outcome of chemotherapy for breast cancer patients. Personalized treatment through use of specific tumor biology can potentially reduce unnecessary chemotherapy and is key to precision care for breast cancer [11,12].
The 21-gene recurrence score (RS) assay (Genomic Health, Inc., Redwood City, CA) employs reverse-transcriptase polymerase chain reaction to assess the expression of 16 related genes and five reference genes from breast cancer tissue, and reports an RS providing a stratification of long-term distant relapse risk and to determine the survival benefit of chemotherapy receipt [13,14]. The current NCCN guidelines have stated that 21-gene RS testing can be considered in N0 and one to three positive lymph nodes (N1) disease to guide the decision for adjuvant therapy [8]. The 21-gene RS assay is also recommended in patients with N1mi disease. However, limited studies are available to assess whether the 21-gene RS results are useful in guiding decisions regarding chemotherapy beyond standard clinicopathologic characteristics [15]. In light of this, we performed a population-based study to assess the predictive and prognostic value of the 21-gene RS assay on decision for chemotherapy in N1mi patients.
Materials and Methods1. PatientsThe Surveillance, Epidemiology, and End Results (SEER) program is a population-based cancer registry maintained by the National Cancer Institute. It records cancer incidence, the first course of treatment, and vital status for approximately 28% of the population of the United States. The SEER 18 Regs (Excl AK) Custom Data Malignant Breast (with Oncotype DX and Additional Treatment Fields) dataset was released in 2017, which including Oncotype DX related variables for invasive breast cancer patients diagnosed between 2004 and 2015 [16]. We have obtained permission to access the SEER database (Authorization Code: 11025-Nov2016). The SEER was queried to identify patients diagnosed with stage T1-2 (tumor size ≤ 5 cm), N1mi, and estrogen receptor (ER)–positive breast invasive ductal carcinoma between 2004 and 2015. The subtypes of invasive ductal carcinoma including infiltrating duct carcinoma not otherwise specified (8,500 /3), infiltrating duct and lobular carcinoma (8,522/3), and infiltrating duct mixed with other types of carcinoma (8,523/3), according to the International Classification of Diseases for Oncology, 3rd edition. Patients with no positive pathology diagnosis, as well as those with data unavailable for race/ethnicity, progesterone receptor (PR) status, tumor grade, and/or surgical procedure were excluded.
2. Patient variablesThe following demographic and clinicopathological variables were included for analysis: year of diagnosis, age, race/ethnicity, tumor size, grade, PR status, surgical procedure, radiotherapy, chemotherapy, traditional RS cutoffs, and Trial Assigning Individualized Options for Treatment (TAILORx) RS cutoffs. All patients were staged using the American Joint Committee on Cancer system 6th edition, those with nodal spread limited to micrometastases no larger than 2 mm were defined as N1mi stage. Traditional RS cutoffs was classified as low-risk (RS < 18), intermediate-risk (RS 18-30), or high-risk (RS > 30) [17], and the optimized RS cut-offs (TAILORx RS cutoffs) were classified into low-risk (RS < 11), intermediate-risk (RS 11-25), and high-risk (RS > 25) groups [18]. Data on human epidermal growth factor receptor-2 (HER2) status began to be recorded in 2010. Therefore, we only assessed the HER2 data after 2010. The primary endpoint of this study was breast cancer-specific survival (BCSS).
3. Statistical analysisThe patient variables between treatment arms were compared using chi-square test. Predict factors associated with chemotherapy receipt were assessed using binomial logistic regression. BCSS was evaluated using Kaplan-Meier methods and the log-rank test for patients diagnosed between 2004 and 2012. Multivariable Cox regression was performed to assess for variables that had an impact on BCSS in patients diagnosed between 2004 and 2012. We did not include the HER2 status in the prognostic analysis. All analyses were performed using IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY). A p-value < 0.05 was considered statistically significant.
ResultsWe identified 4,758 patients. Patient characteristics are presented in Table 1. Fig. 1 shows the patient selection flowchart of this study. The majority of patients were diagnosed after 2010 (n=3,875, 81.4%), aged ≥ 50 years (n=3,636, 76.4%), non-Hispanic white (n=3,537, 74.3%), moderately or poorly/undifferentiated disease (n=3,492, 73.4%), T1 category (n=3,455, 72.6%), and PR-positive status (n=4,418, 92.9%). In 3,853 patients with HER2 status available, most patients were HER2 negative disease (n=3,788, 98.3%). The median RS in this study was 16 (range, 0 to 81). In the traditional RS cutoffs, a total of 2,831 (59.5%), 1,634 (34.3%), and 293 (6.2%) patients were in the low-, intermediate-, and high-risk RS groups, respectively, while 22.2% (n=1,057), 64.6% (n=3,076), and 13.1% (n=625) were in the TAILORx RS cutoffs, respectively.
We included 1,403 patients (29.5%) treated with adjuvant chemotherapy, and 70.5% (n=3,355) of patients did not receive adjuvant chemotherapy. Patient characteristics differed significantly depending on whether chemotherapy was given (Table 1). Patients with diagnosis at later years (p < 0.001), younger age (p < 0.001), poorly/undifferentiated disease (p < 0.001), T2 category (p < 0.001), PR-negative disease (p < 0.001), no receipt of radiotherapy (p=0.007), and higher RS were more likely to receive chemotherapy. In the traditional RS cutoffs, 14.0%, 47.7%, and 77.8% of patients in the low-, intermediate-, and high-risk RS groups received chemotherapy, respectively, and 10.6%, 27.0%, and 73.4% of patients in the TAILORx RS cutoffs received chemotherapy, respectively. The percentage of chemotherapy receipt in different 21-gene RS groups by year of diagnosis is presented in Fig. 2. The results of binomial regression analysis showed that year of diagnosis, age at diagnosis, tumor grade, traditional RS cutoffs, and TAILORx RS cutoffs were independent predictors for chemotherapy receipt (Table 2).
We only included patients diagnosed between 2004 and 2012 to analyze prognostic factors related to BCSS (n=2,599) (Table 3). With a median follow-up of 58 months (range, 0 to 142 months), the traditional RS cutoffs (hazard ratio [HR], 2.774; 95% confidence interval [CI], 1.823 to 4.221; p < 0.001) and TAILORx RS cutoffs (HR, 4.748; 95% CI, 2.752 to 8.193; p < 0.001) were both independent prognostic factors related to BCSS in the traditional RS cutoffs and TAILORx cutoffs multivariate Cox prognostic models, respectively. However, the traditional RS cutoffs (HR, 1.174; 95% CI, 0.654 to 2.109; p=0.591) were not independently associated with BCSS after the traditional RS cutoffs and TAILORx cutoffs were both included in the multivariate analysis, while TAILORx RS cutoffs (HR, 4.748; 95% CI, 2.752 to 8.193; p < 0.001) remained an independent prognostic indicator related to BCSS. In the traditional RS cutoffs, the 5-year BCSS rates were 99.3%, 97.4%, and 91.9% in patients with low-, intermediate-, and high-risk RS groups, respectively (p < 0.001) (Fig. 3A). In the TAILORx RS cutoffs, the 5-year BCSS rates were 99.4%, 99.1%, and 91.5% in patients with low-, intermediate-, and high-risk RS, respectively (p < 0.001) (Fig. 3B). Chemotherapy receipt was not associated with better BCSS in the three models.
We further assessed the impact of chemotherapy on BCSS in different 21-gene RS groups (Table 4). The results of multivariate Cox analysis showed that using both traditional RS cutoffs and TAILORx RS cutoffs, the receipt of chemotherapy did not correlate with better BCSS in patients with low-, intermediate-, or high-risk RS groups. The BCSS curves by whether chemotherapy was given are shown in Fig. 4A-F. Patients with non-Hispanic Black had poor BCSS (HR, 7.248; 95% CI, 1.317 to 39.893; p=0.023) compared to non-Hispanic White in the low-risk RS group with traditional RS cutoffs. In addition, patients with other race/ethnicity (92.5% of patients were non-Hispanic Asian/Pacific) had poor BCSS (HR, 4.749; 95% CI, 1.454 to 15.512; p=0.010) compared to non-Hispanic White in the intermediate-risk RS group with TAILORx RS cutoffs.
DiscussionIn this study, we assessed the impact of 21-gene RS testing in chemotherapy decision for patients with stage N1mi breast cancer. Our results indicate that similar to patients with N0 breast cancer, 21-gene RS assay results are also associated with the percentage of chemotherapy receipt in N1mi breast cancer. However, the outcome may not improve among patients in the high-risk RS group.
There was a significant difference in the percentage of chemotherapy receipt among patients with N1mi disease. In a study from the National Cancer Database, 56.5% of patients were treated with chemotherapy after mastectomy [19]. However, in one study from Sweden, only 24.4% of patients with N1mi disease received chemotherapy [9]. In our study, 29.5% of patients received chemotherapy, and these patients tended to be younger, diagnosed in early years, had more high-grade tumors, and had a higher RS compared to those patients not treated with chemotherapy. The rates of chemotherapy receipt among the low-risk RS group decreased over the study period. In our study, 59.5%, 34.3%, and 6.2% of patients were in the low-, intermediate-, and high-risk RS groups using the traditional RS cutoffs, respectively, which was similar to patients with N0 and N1 disease [20,21]. In addition, 14.0%, 47.7%, and 77.8% of patients received chemotherapy in the low-, intermediate-, and high-risk RS groups using the traditional RS cutoffs, respectively, which was also similar to the distribution observed among N0 and N1 patients, providing support for the similarity of breast cancer gene expression levels in these patient populations [20]. Our findings indicate that patients with high RS results were more likely to receive chemotherapy compared to those with a low RS. These findings suggest that 21-gene RS test results also have clinical utility in guiding chemotherapy decision for N1mi patients.
Several previous studies have indicated that there is no significant difference in survival outcomes between patients with N1mi disease and those with N0 disease [5,6,7,22]. However, other studies provide evidence that N1mi status confers a worse prognosis compared to patients with N0 disease [9,10], and adjuvant therapy may improve disease-free survival [23]. Our results indicate that 21-gene RS testing may also provide prognostic information for patients with N1mi disease. Higher RS were related to a higher risk of breast cancer-related mortality. A study from a large prospectively designed registry also showed that the 5-year distant relapse rate in low-, intermediate-, and high-risk RS was 1.2%, 8.1%, and 26.4%, respectively [24]. If various 21-gene RS groups reflect differing biological behavior among patients with N1mi disease, it may be possible to promote better outcomes through administration of chemotherapy to a high-risk RS group with worsening prognosis.
There is a paucity of prospective data to address the value of chemotherapy in N1mi patients. A study from the Netherlands showed that adjuvant therapy may improve disease-free survival. However, only a limited number of patients were treated with chemotherapy, and most of them received hormonal therapy [23]. A recent study from the National Cancer Database found that receipt of chemotherapy was related to better overall survival (HR, 0.55; 95% CI, 0.48 to 0.64; p < 0.001) [19]. However, there was potential selection bias because the results of BCSS were not reported. In our study, we only included patients with favorable clinicopathological features including small tumor size and ER-positive disease, and we did not find that receipt of chemotherapy was associated with better BCSS in patients with N1mi disease. An American Society of Clinical Oncology (ASCO) panel also indicated that chemotherapy may not be beneficial or required for patients with HER2-negative tumors with micrometastatic nodal disease [25].
In patients with N0 and N1 disease, several studies have found that chemotherapy receipt was associated with better outcomes among patients in the high-risk RS group, but was not related to better outcomes in low- and intermediate-risk RS groups [13,14]. However, our study could not identify a specific 21-gene RS subgroup which may benefit from chemotherapy. The results were similar among patients with traditional RS cutoffs or TAILORx RS cutoffs. In the recommendation of the ASCO panel, chemotherapy may offer no survival benefit in patients with an estimated distant recurrence risk of less than 15% at 10 years using the 21-gene RS test [25]. In our study, even among patients in the high-risk RS group, the 5-year BCSS was 91.9% and 91.5% using traditional RS cutoffs or TAILORx RS cutoffs, respectively, suggesting that patients with high-risk RS may also not benefit from chemotherapy. However, our study could not draw a conclusion regarding the predictive value of the 21-gene RS assay on chemotherapy. More studies are needed to investigate the role of chemotherapy in patients with N1mi disease.
In our study, patients with non-Hispanic Black had poor survival outcome than non-Hispanic White in the low-risk RS group with traditional RS cutoffs, and patients with other race/ethnicity had poor survival outcome compared to non-Hispanic White in the intermediate-risk RS group with TAILORx RS cutoffs. Racial disparities in mortality in different 21-gene RS groups may be related to the racial differences in tumor biology among hormone receptor–positive, HER2-negative breast tumors [26]. Therefore, advancement of our knowledge in the biologic diversity of 21-gene RS testing among different race/ethnicity groups could lead to improvement in survival outcomes.
This study is limited by the retrospective design and the potential for selection bias. Second, we do not have specific information regarding endocrine therapy, endocrine therapy adherence, chemotherapy regimen, the sequence of chemotherapy and surgery, as well as disease recurrence after primary treatment and history of treatment after disease recurrence. However, in the United States, the taxane-based regimens had a sharp increase after 2005, and the majority of patients were receiving taxane-based chemotherapy, especially for patients who received 21-gene RS testing [27]. In addition, the outcomes of our study were similar to previous prospective trials including T1-2N0, ER-positive, and HER2-negative breast cancer patients who received endocrine therapy with or without chemotherapy [18,28], suggesting that most patients also adhered to adjuvant endocrine therapy in our study. Third, HER2 status and the receipt of anti-HER2 therapy were not available in the current SEER database. However, most patients with HER2 status available were HER2 negative disease (98.3%). Therefore, we can assume that the vast majority of patients in our study have their 21-gene RS testing based on clinical practice recommendations [29]. Moreover, as the median follow-up time in our study was only 58 months, longer-term results are needed to draw definitive conclusions on the utilization of 21-gene RS testing in prognostic assessment and chemotherapy decision-making of N1mi disease. Finally, it has been indicated that there are many inaccuracies in the SEER program, with high rates of under-reporting for chemotherapy receipt [30]. However, the primary strength of our study is that we assessed the impact of 21-gene RS testing on chemotherapy decision for N1mi disease, using a large population-based cancer registry.
In conclusion, our study suggests that the 21-gene RS assay does predict prognosis and impact on chemotherapy decision-making in breast cancer patients with N1mi disease. Further study with a large cohort and long-term outcomes is needed to establish the outcome of chemotherapy in N1mi patients by different 21-gene RS groups.
AcknowledgmentsThis work was partly supported by the National Natural Science Foundation of China (81872459, 81803050), Natural Science Foundation of Fujian Province (No. 2016J01635), Natural Science Foundation of Guangdong Province (No. 2018A030313666), and the Science and Technology Planning Projects of Xiamen Science & Technology Bureau (No. 3502Z20174070).
Fig. 1.The patient selection flowchart of this study. RS, recurrence score; ER, estrogen receptor; PR, progesterone receptor. 
Fig. 2.The percentage of chemotherapy receipt by 21-gene recurrence score (RS) groups. (A) Traditional RS cutoffs. (B) Trial Assigning Individualized Options for Treatment (TAILORx) RS cutoffs. 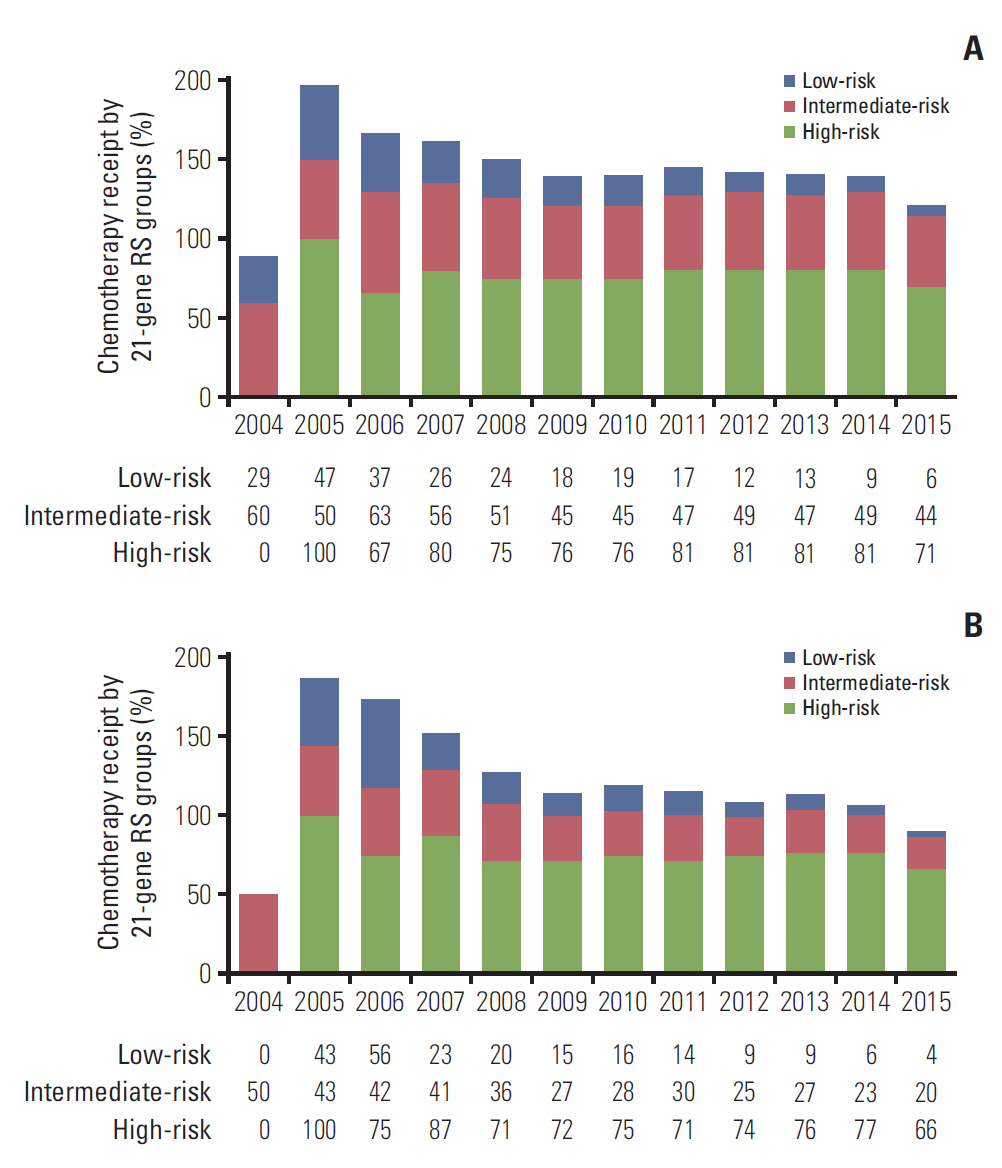
Fig. 3.The breast cancer-specific survival by 21-gene recurrence score (RS) groups. (A) Traditional RS cutoffs. (B) Trial Assigning Individualized Options for Treatment (TAILORx) RS cutoffs. 
Fig. 4.The association of chemotherapy with breast cancer-specific survival by 21-gene recurrence score (RS) groups. Traditional RS cutoffs: low-risk (A), intermediate-risk (B), and high-risk (C). Trial Assigning Individualized Options for Treatment (TAILORx) RS cutoffs: low-risk (D), intermediate-risk (E), and high-risk (F). 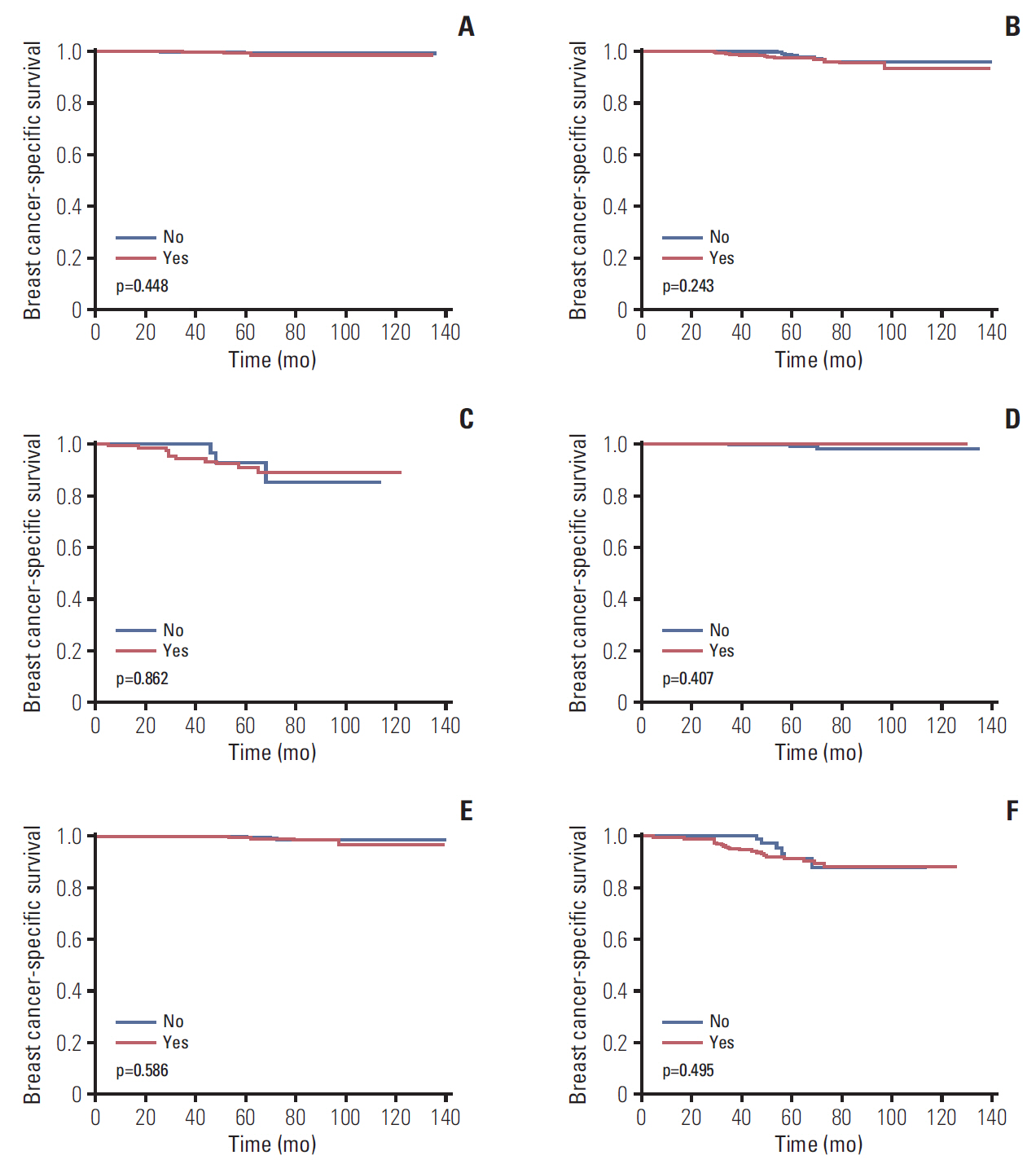
Table 1.Association between patient characteristics and chemotherapy receipt Table 2.Predictors of chemotherapy receipt Table 3.Multivariate prognostic analysis of breast cancer-specific survival Table 4.Multivariate prognostic analysis of breast cancer-specific survival by different 21-gene RS groups References1. Maaskant AJ, van de Poll-Franse LV, Voogd AC, Coebergh JW, Tutein Nolthenius-Puylaert MC, Nieuwenhuijzen GA. Stage migration due to introduction of the sentinel node procedure: a population-based study. Breast Cancer Res Treat. 2009;113:173–9.
2. van der Heiden-van der Loo M, Bezemer PD, Hennipman A, Siesling S, van Diest PJ, Bongers V, et al. Introduction of sentinel node biopsy and stage migration of breast cancer. Eur J Surg Oncol. 2006;32:710–4.
3. Salhab M, Patani N, Mokbel K. Sentinel lymph node micrometastasis in human breast cancer: an update. Surg Oncol. 2011;20:e195–206.
4. Peethambaram PP, Hoskin TL, Day CN, Goetz MP, Habermann EB, Boughey JC. Use of 21-gene recurrence score assay to individualize adjuvant chemotherapy recommendations in ER+/HER2– node positive breast cancer-A National Cancer Database study. NPJ Breast Cancer. 2017;3:41.
5. Montagna E, Viale G, Rotmensz N, Maisonneuve P, Galimberti V, Luini A, et al. Minimal axillary lymph node involvement in breast cancer has different prognostic implications according to the staging procedure. Breast Cancer Res Treat. 2009;118:385–94.
6. Hansen NM, Grube B, Ye X, Turner RR, Brenner RJ, Sim MS, et al. Impact of micrometastases in the sentinel node of patients with invasive breast cancer. J Clin Oncol. 2009;27:4679–84.
7. Langer I, Marti WR, Guller U, Moch H, Harder F, Oertli D, et al. Axillary recurrence rate in breast cancer patients with negative sentinel lymph node (SLN) or SLN micrometastases: prospective analysis of 150 patients after SLN biopsy. Ann Surg. 2005;241:152–8.
8. NCCN clinical Practice guidelines in oncology V.2.2018. Breast Cancer [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network; 2018. [cited 2018 Oct 21]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
9. Andersson Y, Bergkvist L, Frisell J, de Boniface J. Long-term breast cancer survival in relation to the metastatic tumor burden in axillary lymph nodes. Breast Cancer Res Treat. 2018;171:359–69.
10. Iqbal J, Ginsburg O, Giannakeas V, Rochon PA, Semple JL, Narod SA. The impact of nodal micrometastasis on mortality among women with early-stage breast cancer. Breast Cancer Res Treat. 2017;161:103–15.
11. De Abreu FB, Schwartz GN, Wells WA, Tsongalis GJ. Personalized therapy for breast cancer. Clin Genet. 2014;86:62–7.
12. Sabatier R, Goncalves A, Bertucci F. Personalized medicine: present and future of breast cancer management. Crit Rev Oncol Hematol. 2014;91:223–33.
13. Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24:3726–34.
14. Albain KS, Barlow WE, Shak S, Hortobagyi GN, Livingston RB, Yeh IT, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11:55–65.
15. Frazier TG, Fox KR, Smith JS, Laronga C, McSwain A, Paul D, et al. A retrospective study of the impact of 21-gene recurrence score assay on treatment choice in node positive micrometastatic breast cancer. Pharmaceuticals (Basel). 2015;8:107–22.
16. Surveillance, Epidemiology, and End Results (SEER) Program. Oncotype DX database (2004-2015) [Internet]. Bethesda, MD: National Cancer Institute; 2018. [cited 2018 Oct 21]. Available from: https://seer.cancer.gov/seerstat/databases/oncotypedx/index.html
17. Wong WB, Ramsey SD, Barlow WE, Garrison LP Jr, Veenstra DL. The value of comparative effectiveness research: projected return on investment of the RxPONDER trial (SWOG S1007). Contemp Clin Trials. 2012;33:1117–23.
18. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379:111–21.
19. Wu SP, Tam M, Shaikh F, Lee A, Chun J, Schnabel F, et al. Post-mastectomy radiation therapy in breast cancer patients with nodal micrometastases. Ann Surg Oncol. 2018;25:2620–31.
20. Turashvili G, Chou JF, Brogi E, Morrow M, Dickler M, Norton L, et al. 21-Gene recurrence score and locoregional recurrence in lymph node-negative, estrogen receptor-positive breast cancer. Breast Cancer Res Treat. 2017;166:69–76.
21. Goodman CR, Seagle BL, Kocherginsky M, Donnelly ED, Shahabi S, Strauss JB. 21-Gene recurrence score assay predicts benefit of post-mastectomy radiotherapy in T1-2 N1 breast cancer. Clin Cancer Res. 2018;24:3878–87.
22. Forissier V, Tallet A, Cohen M, Classe JM, Reyal F, Chopin N, et al. Is post-mastectomy radiation therapy contributive in pN0-1mi breast cancer patients? Results of a French multi-centric cohort. Eur J Cancer. 2017;87:47–57.
23. de Boer M, van Deurzen CH, van Dijck JA, Borm GF, van Diest PJ, Adang EM, et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N Engl J Med. 2009;361:653–63.
24. Stemmer SM, Steiner M, Rizel S, Geffen DB, Nisenbaum B, Peretz T, et al. Clinical outcomes in ER+ HER2 -node-positive breast cancer patients who were treated according to the Recurrence Score results: evidence from a large prospectively designed registry. NPJ Breast Cancer. 2017;3:32.
25. Henry NL, Somerfield MR, Abramson VG, Allison KH, Anders CK, Chingos DT, et al. Role of patient and disease factors in adjuvant systemic therapy decision making for earlystage, operable breast cancer: American Society of Clinical Oncology Endorsement of Cancer Care Ontario Guideline Recommendations. J Clin Oncol. 2016;34:2303–11.
26. D'Arcy M, Fleming J, Robinson WR, Kirk EL, Perou CM, Troester MA. Race-associated biological differences among Luminal A breast tumors. Breast Cancer Res Treat. 2015;152:437–48.
27. Giordano SH, Lin YL, Kuo YF, Hortobagyi GN, Goodwin JS. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30:2232–9.
28. Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–14.
|
|
||||||||||||||||||||||||||||||||||||||||||||