AbstractPurposeThis study was conducted to evaluate the impact of supraclavicular lymph node radiotherapy (SCNRT) on N1 breast cancer patients receiving post-lumpectomy whole-breast irradiation (WBI) and anthracycline plus taxane-based (AT) chemotherapy.
Materials and MethodsWe performed a case-control analysis to compare the outcomes of WBI and WBI plus SCNRT (WBI+SCNRT). Among 1,147 patients with N1 breast cancer who received post-lumpectomy radiotherapy and AT-based chemotherapy in 12 hospitals, 542 were selected after propensity score matching. Patterns of failure, disease-free survival (DFS), distant metastasis-free survival (DMFS), and treatment-related toxicity were compared between groups.
ResultsA total of 41 patients (7.6%) were found to have recurrence. Supraclavicular lymph node (SCN) failure was detected in three patients, two in WBI and one in WBI+SCNRT. All SCN failures were found simultaneously with distant metastasis. There was no significant difference in patterns of failure or survival between groups. The 5-year DFS and DMFS for patients with WBI and WBI+SCNRT were 94.4% versus 92.6% (p=0.50) and 95.1% versus 94.5% (p=0.99), respectively. The rates of lymphedema and radiation pneumonitis were significantly higher in the WBI+SCNRT than in the WBI.
IntroductionPost-mastectomy radiotherapy (PMRT) of the regional lymph nodes and chest wall is associated with reduced locoregional recurrence and improved survival in patients with node-positive breast cancer [1]. Based on the effects of PMRT, post-lumpectomy regional nodal irradiation (RNI) is recommended to be added to whole-breast irradiation (WBI) for patients with a large tumor burden in the axillary lymph nodes (ALN), such as in cases with four or more ALN metastases [2]. However, for patients with one to three positive ALNs (N1), it remains uncertain whether RNI improves disease outcome in a post-lumpectomy setting. Recently, two randomized trials reported that adding RNI to WBI reduced breast cancer recurrence relative to WBI alone in patients with early breast cancer [3,4]. However, the systemic agents adopted in the two studies were considered less effective than the current standard. Therefore, it is necessary to reassess the benefits of RNI for N1 breast cancer in patients treated with modern systemic treatments [5].
Regarding the extent of RNI for N1 breast cancer, it is not yet known which regional lymph nodes should be included in post-lumpectomy radiotherapy. In the aforementioned trials and studies of PMRT [1], all regional lymph nodes including ALN, the internal mammary lymph node (IMN), and the supraclavicular lymph node (SCN) were covered for RNI. After WBI alone, SCN metastasis occurred in about 0.9%-9.2% of patients with N1 breast cancer [6-9]. Given the risk of SCN recurrence after WBI, it has been proposed that prophylactic SCN radiotherapy (SCNRT) is necessary after WBI for patients with N1 breast cancer [9-11]. However, the rates of SCN recurrence were estimated based on studies adapting less effective systemic treatments [9,10]. Moreover, no studies have specifically evaluated the prognostic significance of SCNRT in N1 breast cancer. To determine if adding SCNRT is necessary in post-lumpectomy radiotherapy for N1 breast cancer, the benefits of elective SCNRT in patients undergoing effective systemic treatments must be analyzed.
The present study was conducted to investigate the prognostic impact of elective SCNRT in N1 breast cancer patients who received systemic treatments, including anthracycline plus taxane-based (AT) chemotherapy. We compared treatment outcomes and complications between the two treatment groups, WBI alone versus WBI plus SCNRT (WBI+SCNRT), to determine if elective SCNRT is beneficial for N1 breast cancer patients in an era of effective systemic treatments.
Materials and Methods1. Study design and patientsTo compare treatment outcomes between groups, WBI alone versus WBI+SCNRT, we conducted a matched casecontrol study of patients with N1 breast cancer using patient data from 12 hospitals that are members of the Korean Radiation Oncology Group (KROG). Patients who underwent AT chemotherapy and post-lumpectomy radiotherapy for N1 breast cancer between January 2006 and December 2010 were included in this study. The inclusion criteria were patients with N1 breast cancer who received breast conservingsurgery (BCS) and axillary lymph node dissection (ALND), those who completed postoperative AT chemotherapy and radiotherapy as planned, and those for whom information regarding pathological features of the tumor was available. The exclusion criteria were patients who received neoadjuvant chemotherapy, chemotherapy other than AT, or IMN radiotherapy. The Institutional Review Board of each participating hospital approved the current study.
The collected patient data were pathologic features of each tumor such as tumor size, number of positive lymph nodes, histologic grade (HG), presence of lymphovascular invasion (LVI), and expression status of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2). ER/PR positivity was defined as a 3 to 8 Allred score by immunohistochemistry (IHC). HER2 positivity was defined as either staining 3+ by IHC or 2+ by IHC with positive fluorescence in situ hybridization or chromogenic in situ hybridization. The molecular subtype of each breast cancer was categorized as follows: ER+ or PR+, HER2–, and HG 1 or 2 (i.e., luminal A); ER+ or PR+, HER2–, and HG3 (i.e., luminal B); ER+ or PR+, HER2+ (i.e., luminal HER2); ER–, PR–, and HER2+ (i.e., HER2 enriched); ER–, PR–, and HER2– (i.e., triple negative).
2. TreatmentsAll patients received BCS and ALND with or without sentinel lymph node evaluation. Doxorubicin and cyclophosphamide (AC) or epirubicin and cyclophosphamide (EC) followed by paclitaxel or docetaxel (T) were prescribed to all patients. According to the hormonal receptor or HER2 positivity of each tumor, adjuvant endocrine therapy or anti-HER2 agent was administered. WBI and tumor bed boost were given to all patients. The decision regarding whether to administer elective SCNRT to patients was made according to institutional policies across the 12 participating hospitals. Pathologic features such as high HG, positive LVI, large numbers of metastatic lymph nodes, or non-luminal subtypes were high-risk factors that influence the decision of whether to add SCNRT to WBI.
The doses for whole breast and tumor bed were 45.0-60.4 Gy at 1.8-3.0 Gy per fraction and 4.0-19.8 Gy at 1.8-3.5 Gy per fraction, respectively. Conventionally fractionated WBI with a daily dose of 1.8-2.0 Gy was performed in 512 patients (94.5%), while hypofractionated WBI with a total dose of 51.0 Gy in 17 fractions at 3.0 Gy per fraction was delivered to 30 patients (5.5%). The radiation dose to SCN was 45.0-50.4 Gy at 1.8-2.0 Gy per fraction. Borders of each field of WBI or WBI+SCNRT were variously defined in the 12 hospitals according to each institutional policy. Nevertheless, there were common principles of beam configuration. The superior, inferior, and lateral borders of the field of WBI were 2 cm beyond the palpable breast tissue. The medial border was located at midline, and the superficial border allowed 2 cm of flash beyond the breast. The superior, inferior, lateral, and medial borders of the field of SCNRT were the upper border of the supraclavicular fossa, match line of tangential beams of WBI, lateral edge of clavicle, and 0.5 cm from the spinal cord. ALNs were not intentionally irradiated. Nonetheless, level I and some portion of level II ALNs were covered during WBI while a part of level II and III ALNs and the SCN were irradiated during SCNRT.
Treatment related toxicity was graded by the Common Terminology Criteria for Adverse Events, ver. 3.0 [12].
3. Statistical analysisOverall survival (OS), disease-free survival (DFS), locoregional recurrence-free survival (LRRFS), and distant metastasis-free survival (DMFS) were defined as the interval from surgery to death, cancer recurrence, loco-regional recurrence, and distant metastasis, respectively. Among the variables, number of tumors, LVI, HG, and hormone receptor status were considered as binary variables. Patient age, tumor size, number of positive nodes, and ratio of positive nodes were analyzed as continuous variables. An optimal cut-off of continuous variables was defined using analysis of the area under the curve of receiver operating characteristics. The value for which sensitivity and specificity were the highest was chosen as the optimal cut-off point for each variable. The chi-square test or Fisher exact test was used to compare the patient characteristics between the two groups. Survival probability was estimated using the Kaplan-Meier method, and the log-rank test was used to compare survival between groups with different variables. To determine the independent prognostic factors for the outcomes, Cox regression analysis with stepwise selection was used. A two sided p-value of < 0.05 was assumed as statistically significant.
To maintain a balance of covariates between the two treatment groups, one-to-one matching was performed on the basis of the propensity scores of each patient. As matching variables, we selected tumor size, LVI, HG, and ratio of positive lymph nodes. These variables were identified as significant prognostic factors for patient survival in the primary data set (S1 Table). Propensity score matching was conducted with the R Statistical Software ver. 3.2.3 (The R foundation for Statistical Computing, Vienna, Austria) using the MatchIt package with the nearest-neighbor method. Statistical analyses were performed with the SPSS ver. 22.0 (IBM Corp., Armonk, NY).
ResultsA total of 1,147 patients met the inclusion criteria of the current study. The 5-year rates of DFS, OS, LRRFS, and DMFS of 1,147 patients were 93.0%, 98.5%, 97.3%, and 94.2%, respectively. Among the 1,147 patients, 783 had WBI alone, while 364 received WBI+SCNRT. The 5-year DFS rate was 93.1% for patients with WBI, while it was 92.6% for patients with WBI+SCNRT (p=0.79) (S1 Table). Between the two groups of patients, there were significant differences in variables such as pathology, LVI, molecular subtype, number of positive lymph nodes, ratio of metastatic lymph nodes, endocrine therapy, and anti-HER2 therapy (Table 1). After propensity score matching, a total of 542 patients, 271 in each group, were selected for analysis.
1. Baseline characteristicsThe median age of the patients was 47 years (range, 26 to 69 years). All patients had a clear resection margin on their surgical specimen. The median tumor size was 20 mm (range 0.1 to 51 mm). All but two patients had T1 or T2 stage tumor. The median number of examined lymph nodes was 16 (range, 2 to 48). Among the 414 patients with hormone receptor–positive tumors, 384 (92.8%) were treated with endocrine therapy. In 105 patients with HER2 amplified tumors, anti-HER2 agent was given to 28 (26.7%). Details regarding the patient characteristics are shown in Table 1.
2. Treatment outcomes and toxicityThe median follow-up times of the patients with WBI alone and WBI+SCNRT were 73 months (range, 10 to 111 months) and 60 months (range, 12 to 111 months), respectively. A total of 41 patients (7.6%) were found to have disease recurrence. Patterns of the first failure were not significantly different between the two groups (Table 2). SCN failure was detected in three patients, two in WBI alone and one in WBI+SCNRT. All SCN failures were found simultaneous with distant metastasis. There was no significant difference in patient survival between groups. The 5-year rate of DFS for patients with WBI alone or WBI+ SCNRT was 94.4% and 92.6%, respectively (p=0.50) (Fig. 1). The 5-year OS, LRRFS, and DMFS rates were 99.2%, 98.1%, and 95.1%, respectively, for WBI alone and 97.7%, 96.1%, and 94.5% for WBI+SCNRT (p=0.54, p=0.21, and p=0.99, respectively). During follow-up, contralateral breast cancer was detected in three patients (0.5%), all of whom were treated with WBI alone. Subgroup analysis revealed that the effect of SCNRT on DFS was not different according to prognostic factors (Table 3).
The rates of lymphedema and radiation pneumonitis were significantly higher in patients with SCNRT than in those without SCNRT (Table 4). A total of 10.7% of patients showed lymphedema after WBI alone, whereas 16.6% of patients presented lymphedema after WBI+SCNRT. Radiation-related pneumonitis was found in 0.7% of patients after WBI alone, while it was detected in 4.1% of patients with WBI+SCNRT.
DiscussionIn this case-control study, we evaluated the prognostic impact of elective SCNRT in post-lumpectomy radiotherapy for N1 breast cancer. We found that there was no benefit of the addition of SCNRT in patients treated with contemporary systemic treatments including AT chemotherapy. Treatment outcomes with respect to loco-regional and distant tumor control were not significantly different between the WBI alone group and the WBI+SCNRT group. The addition of SCNRT to WBI was associated with increased risk of lymphedema and radiation-related pneumonitis compared with WBI alone. Therefore, we suggest that elective SCNRT is not an essential component in post-lumpectomy radiotherapy for N1 breast cancer in patients receiving AT-based chemotherapy.
In patients with early breast cancer, RNI is added to WBI to control microscopic regional nodal disease and prevent systemic spread of cancer by sterilizing subclinical disease in the regional lymph nodes [13]. Because the regional lymph nodes may be the only reservoir of residual disease in some patients, eradication of the reservoir with elective RNI is expected to improve survival in selected patients with breast cancer [14]. Two randomized studies compared treatment outcomes between the WBI alone group and the WBI plus RNI including ALN, IMN, and SCN [3,4]. The European Organization for Research and Treatment of Cancer (EORTC) 22922-10925 trial was conducted in patients with N0-N3 breast cancer after BCS or mastectomy [3]. Patients with N1 breast cancer accounted for 43.1% of the study population, and BCS was conducted in 76.1% of the patients. Chemotherapy and hormone therapy were administered to 54% and 59% of the patients, respectively. The results revealed that the 10-year DFS rate was significantly higher in the group receiving RNI than in the group without RNI (72.1% vs. 69.1%, p=0.04). Another randomized trial, the National Cancer Institute of Canada MA.20, enrolled patients who had undergone BCS. In this study, 84.9% of the all patients had N1 breast cancer [4], among which 60% received anthracycline, while 25% were treated with anthracycline plus taxane. As with the EORTC study, the authors of the MA.20 trial found that the addition of RNI significantly improved the 5-year DFS compared to WBI alone (89.7% vs. 84.0%, p=0.003) [4,15]. Nonetheless, the benefit of RNI found in the aforementioned studies might be attributed to incorporation of less effective systemic treatments. Systemic treatments now known to improve loco-regional control, such as taxane or endocrine therapy, were prescribed to a small percentage of patients in the studies. Moreover, the results of the previous two studies could not be used to determine the advantages specific to SCNRT because all regional lymphatics were irradiated in those studies.
A large portion of the lymphatics from the breast pass through the ALN to SCN or drain to the IMN. There is direct nodal drainage to the SCN without traversing the ALN [16,17]. Generally, less than 10% of patients with N1 breast cancer experienced SCN failure after WBI alone [6,7,9-11]. According to a previously conducted survey, about half of the EORTC-affiliated radiation oncology centers advocate SCNRT for patients with N1 breast cancer [18]. In Canada, 64% of N1 breast cancer patients were treated with SCNRT in addition to post-lumpectomy radiotherapy [19]. Even with suggestions to include elective SCNRT in N1 breast cancer treatment, to our knowledge, no study has specifically tested the effects of SCNRT. Most studies reporting SCN recurrence adopted cyclophosphamide, methotrexate, fluorouracil or anthracycline–based chemotherapy for adjuvant treatment of N1 breast cancer (Table 5). Endocrine therapy was not generally administered to these patients. Because the regimens of systemic treatments used in the studies are now considered suboptimal [5], the rates of SCN recurrence and the benefit of SCNRT in N1 breast cancer must be reevaluated in the context of modern systemic treatments.
In this study, we assessed the benefit of elective SCNRT in N1 breast cancer patients treated with the current standard systemic treatments. All patients were given AT-based chemotherapy, and over 92% of patients with hormoneresponsive breast cancer received adjuvant endocrine therapy. We found that, regardless of elective SCNRT, SCN metastases occurred in less than 1% of patients with N1 breast cancer when they were treated with post-lumpectomy WBI, AT-based chemotherapy, and systemic agents according to the molecular subtype of the tumor. All SCN failures were found simultaneously with distant sites metastases. Moreover, we did not observe any significant change in patient survival or pattern of the first failure in response to the addition of SCNRT. According to previous studies, administering AT-based chemotherapy reduced disease recurrence and breast cancer mortality more effectively than applying an anthracycline-based regimen alone for patients with early breast cancer [20,21]. The addition of taxane to AC resulted in significant improvement in DFS compared to AC alone in patients with node-positive breast cancer [22]. Notably, the incidence of loco-regional recurrence was significantly reduced by the addition of taxane [23]. The relative benefit of taxane-containing chemotherapy to locoregional control is reported to be around 20%-30% [5]. According to a previous report, the 5-year locoregional relapse rate was 9.7% in the AC treated group, while it was 3.7% in patients receiving AC and taxane chemotherapy [23]. Endocrine therapy and anti-HER2 treatment reduce loco-regional recurrence by about 50% when they are properly conducted according to the molecular subtype of the tumor [5,24]. Given the effectiveness of the systemic treatments in the current study, it is likely that applying AT-based chemotherapy to all patients and administering endocrine treatment to most of the patients contributed to the absence of gain by elective SCNRT. In the MA.20 trial, where N1 breast cancer accounted for 84.9% of the enrolled cases, the 5-year DFS rate was reported as 92.4% in patients with WBI plus RNI [15]. In the present study, the 5-year DFS rate of patients with WBI alone was 94.4%. Even if it is difficult to directly compare the results of our current study to those of the MA.20 trial, it seems that the outcome of WBI alone is comparable to the results of WBI plus RNI when the WBI was administered coupled with effective systemic treatments. In a study by Yates et al. [10], the authors examined the risk of SCN failure in patients with N1 breast cancer after WBI alone over a 25-year period at two hospitals in the UK. Between 1975 and 2000, the 5-year SCN recurrence rate fell from 7.3% to 2.9% [10], during which time the use of chemotherapy and endocrine therapy increased. These findings suggest that the improved effect of systemic therapies during the 25-year period influenced regional tumor control of N1 breast cancer. Similarly, the impact of systemic treatments should be considered when determining the field of radiotherapy for N1 breast cancer. In particular, the effect of AT chemotherapy on loco-regional control of N1 breast cancer should be accounted for when optimizing the RNI field.
There is a possibility that microscopic tumor burden in the SCN area is not sufficient to bring benefits by the addition of SCNRT in N1 breast cancer. According to a study describing patterns of lymphatic drainage of breast cancer by sentinel lymph node mappings, only 0.5% of patients with clinically node-negative breast cancer had sentinel lymph node metastasis in the SCN area [17]. Likewise, patients with N1 breast cancer might have minimal tumor burdens in the SCN region. Given the low amount of subclinical disease in SCN, elective SCNRT did not have an advantage over WBI alone in patients with N1 breast cancer.
We found that lymphedema and pneumonitis occurred more frequently after WBI+SCNRT than after WBI alone. In the current study, about 16% of patients showed lymphedema after WBI+SCNRT. ALND, which was performed on all patients in this study, might contribute to the risk of lymphedema. Previous studies reported that arm edema was found in 3%-25% of patients with ALND, WBI, or SCNRT [13]. Even when the complications were not severe, applying SCNRT was significantly associated with an increase in adverse events. Therefore, the adverse effects caused by the addition of SCNRT should be considered when determining the radiation field for N1 breast cancer.
It should be noted that our study had several limitations. Specifically, there might have been biases in selecting patients because patient data were retrospectively collected and matched in this study. We balanced probable prognostic factors of patients between the two treatment groups by matching propensity score; however, unperceived variables might have been unevenly distributed between groups. Additionally, pathologic examinations were conducted at several different hospitals in this study, thereby causing missing information regarding some pathologic characteristics of the tumors. For example, not all participating hospitals were able to provide information describing extracapsular extensions of metastatic lymph nodes or Ki-67 levels. Therefore, it is possible that the pathologic variables could have been arranged unequally between the treatment groups. Finally, the duration of follow-up of patients was relatively short in this study. The median follow-up period was 73 months for patients with WBI and 60 months for those with WBI+SCNRT. It has been reported that long-term follow-up is necessary for patients with breast cancer to detect late disease recurrence and treatment-related adverse effects [25]. Therefore, further follow-up of patients is needed to weigh benefits against adverse effects of the addition of SCNRT. Accordingly, a randomized trial to confirm the prognostic impact of SCNRT in N1 breast cancer is warranted.
In this study, we could not determine a subgroup of patients who benefitted from elective SCNRT. There were no pathologic features or molecular subtypes significantly associated with improved outcome by the addition of SCNRT. It was recently reported that the gene expression profile of a tumor can predict loco-regional recurrence and distant metastasis of breast cancer [26,27]. Similarly, the biological characteristics of tumors are expected to help in selecting patients with N1 breast cancer who can achieve therapeutic gain by the addition of SCNRT.
In conclusion, elective SCNRT did not provide an advantage for tumor control in patients with N1 breast cancer when they received effective systemic treatments. Mild treatment-related complications were found more frequently following the addition of SCNRT. Further randomized studies are necessary to determine the optimal field of post-lumpectomy radiotherapy for N1 breast cancer.
Electronic Supplementary MaterialSupplementary materials are available at Cancer Research and Treatment website (http://www.e-crt.org).
Fig. 1.Survival according to radiation field. Disease-free survival (A), loco-regional recurrence-free survival (LRRFS) (B), distant metastasis-free survival (DMFS) (C), and overall survival (D) are shown. WBI, whole-breast irradiation; SCNRT, supraclavicular lymph node radiotherapy. 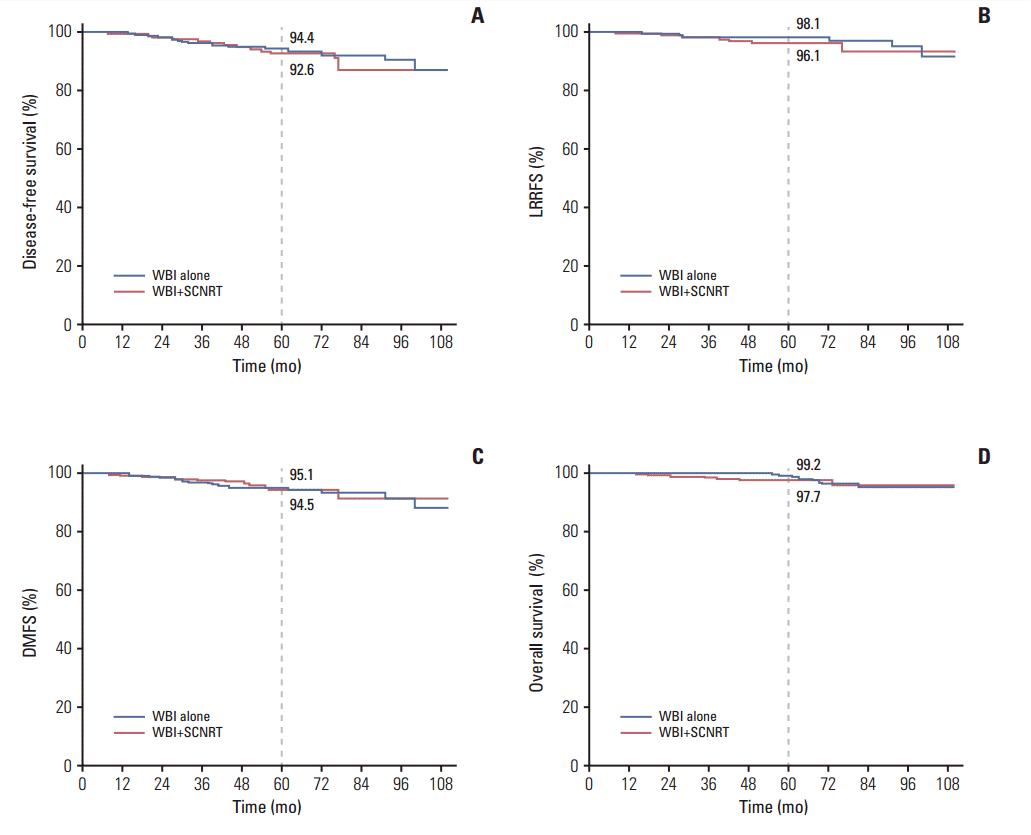
Table 1.Comparison of patient characteristics between groups
Values are presented as number (%). WBI, whole-breast irradiation; SCNRT, supraclavicular radiotherapy; IDC, invasive ductal carcinoma; LVI, lymphovascular invasion; HG, histologic grade; HER-2, human epidermal growth factor receptor-2; LN, lymph node. Table 2.Patterns of the first failure according to field of radiotherapy
Values are presented as number (%). WBI, whole-breast irradiation; SCNRT, supraclavicular radiotherapy. a) Regional recurrence occurred in the axillary lymph node (n=1) in WBI alone and the internal mammary lymph node (n=3) in WBI+SCNRT. Supraclavicular lymph node failure was detected in three patients, two in WBI alone and one in WBI+SCNRT. All supraclavicular lymph node failures were found simultaneously with distant metastasis. Table 3.DFS according to patient and tumor characteristics between WBI alone and WBI+SCNRT
Table 4.Treatment-related toxicities
Table 5.Studies reporting the incidence of SCN metastasis after whole-breast radiotherapy with or without elective SCN irradiation in patients with N1 breast cancer
SCN, supraclavicular lymph node; HTx, hormone therapy; CTx, chemotherapy; RT, radiotherapy; CMF, cyclophosphamide, methotrexate, and 5-fluorouracil; WBI, whole-breast irradiation; NR, not reported; SCNFFS, supraclavicular lymph node failure-free survival; SCNRT, supraclavicular radiotherapy; LRRFS, loco-regional recurrence-free survival; AC, adriamycin and cyclophosphamide; FAC, 5-fluorouracil, adriamycin, and cyclophosphamide; DMFS, distant metastasis failure-free survival; AT, anthracycline with taxane; RNI, regional-nodal irradiation (internal mammary, supraclavicular, and axillary lymph nodes); DFS, disease free survival; CWI, chest wall irradiation. a) The study included 4,185 patients with N0 (68.6%), N1 (19.7%), N2 (9.3%), or unknown nodal status (2.4%) breast cancer. The proportions indicate the number of patients who underwent hormone therapy or chemotherapy among all patients, b) There were 469 patients (37%) treated with hormone therapy alone, 408 patients (33%) treated with chemotherapy alone, and 340 patients (27%) treated with both hormone therapy and chemotherapy, c) The proportion of patients receiving hormone therapy and chemotherapy increased with time. The rate of SCN failure steadily decreased over the same time period, References1. EBCTCG (Early Breast Cancer Trialists' Collaborative Group), McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: metaanalysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–35.
2. Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, et al. Tailoring therapies: improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol. 2015;26:1533–46.
3. Poortmans PM, Collette S, Kirkove C, Van Limbergen E, Budach V, Struikmans H, et al. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med. 2015;373:317–27.
4. Whelan TJ, Olivotto IA, Parulekar WR, Ackerman I, Chua BH, Nabid A, et al. Regional nodal irradiation in early-stage breast cancer. N Engl J Med. 2015;373:307–16.
5. Mannino M, Yarnold JR. Local relapse rates are falling after breast conserving surgery and systemic therapy for early breast cancer: can radiotherapy ever be safely withheld? Radiother Oncol. 2009;90:14–22.
6. Livi L, Paiar F, Simontacchi G, Barca R, Detti B, Fondelli S, et al. Loco regional failure pattern after lumpectomy and breast irradiation in 4,185 patients with T1 and T2 breast cancer: implications for nodal irradiation. Acta Oncol. 2006;45:564–70.
7. Reddy SG, Kiel KD. Supraclavicular nodal failure in patients with one to three positive axillary lymph nodes treated with breast conserving surgery and breast irradiation, without supraclavicular node radiation. Breast J. 2007;13:12–8.
8. Galper S, Recht A, Silver B, Manola J, Gelman R, Schnitt SJ, et al. Factors associated with regional nodal failure in patients with early stage breast cancer with 0-3 positive axillary nodes following tangential irradiation alone. Int J Radiat Oncol Biol Phys. 1999;45:1157–66.
9. Yu JI, Park W, Huh SJ, Choi DH, Lim YH, Ahn JS, et al. Determining which patients require irradiation of the supraclavicular nodal area after surgery for N1 breast cancer. Int J Radiat Oncol Biol Phys. 2010;78:1135–41.
10. Yates L, Kirby A, Crichton S, Gillett C, Cane P, Fentiman I, et al. Risk factors for regional nodal relapse in breast cancer patients with one to three positive axillary nodes. Int J Radiat Oncol Biol Phys. 2012;82:2093–103.
11. Truong PT, Jones SO, Kader HA, Wai ES, Speers CH, Alexander AS, et al. Patients with t1 to t2 breast cancer with one to three positive nodes have higher local and regional recurrence risks compared with node-negative patients after breast-conserving surgery and whole-breast radiotherapy. Int J Radiat Oncol Biol Phys. 2009;73:357–64.
12. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Bethesda, MD: U.S. Department of Health and Human Services; 2009.
13. Xie L, Higginson DS, Marks LB. Elective regional nodal irradiation in patients with early-stage breast cancer. Semin Radiat Oncol. 2011;21:66–78.
14. Jagsi R. Progress and controversies: radiation therapy for invasive breast cancer. CA Cancer J Clin. 2014;64:135–52.
15. Whelan TJ, Olivotto I, Ackerman I, Chapman JW, Chua B, Nabid A, et al. NCIC-CTG MA.20: an intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol. 2011;29 Suppl:LBA1003.
16. Tanis PJ, Nieweg OE, Valdes Olmos RA, Kroon BB. Anatomy and physiology of lymphatic drainage of the breast from the perspective of sentinel node biopsy. J Am Coll Surg. 2001;192:399–409.
17. Estourgie SH, Nieweg OE, Olmos RA, Rutgers EJ, Kroon BB. Lymphatic drainage patterns from the breast. Ann Surg. 2004;239:232–7.
18. Belkacemi Y, Kaidar-Person O, Poortmans P, Ozsahin M, Valli MC, Russell N, et al. Patterns of practice of regional nodal irradiation in breast cancer: results of the European Organization for Research and Treatment of Cancer (EORTC) NOdal Radiotherapy (NORA) survey. Ann Oncol. 2015;26:529–35.
19. Wai ES, Lesperance M, Speers CH, Truong PT, Jones S, Tyldesley S, et al. Increased use of regional radiotherapy is associated with improved outcome in a population-based cohort of women with breast cancer with 1-3 positive nodes. Radiother Oncol. 2010;97:301–6.
20. Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–44.
21. Qin YY, Li H, Guo XJ, Ye XF, Wei X, Zhou YH, et al. Adjuvant chemotherapy, with or without taxanes, in early or operable breast cancer: a meta-analysis of 19 randomized trials with 30698 patients. PLoS One. 2011;6:e26946
22. Mamounas EP, Bryant J, Lembersky B, Fehrenbacher L, Sedlacek SM, Fisher B, et al. Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol. 2005;23:3686–96.
23. Sartor CI, Peterson BL, Woolf S, Fitzgerald TJ, Laurie F, Turrisi AJ, et al. Effect of addition of adjuvant paclitaxel on radiotherapy delivery and locoregional control of node-positive breast cancer: cancer and leukemia group B 9344. J Clin Oncol. 2005;23:30–40.
24. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–84.
25. Cuzick J. Statistical controversies in clinical research: longterm follow-up of clinical trials in cancer. Ann Oncol. 2015;26:2363–6.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||