Introduction
Chronic inflammation enhances cell proliferation. This microenvironment potentiates and promotes neoplastic risk [1]. Epidemiological studies have shown that chronic inflammation predisposes individuals to various types of cancer [1-3]. Cancer-related inflammation affects many aspects of malignancy, including proliferation and survival of malignant cells, angiogenesis, and therapeutic response [3]. In a previous study that utilizes a mouse model [4], we showed that an inflammatory response is elicited in the early stages of the postsurgical wound healing process, leading to an increase in the number of inflammatory cells in the peritoneum. This further increases pro-matrix metalloproteinase-9 (pro-MMP-9), which plays a key role in the growth and progression of peritoneal metastases. Based on these experimental results, we hypothesize that the local metastatic microenvironment will be changed during the early inflammatory phase in the process of wound healing. Clinically, there have been efforts to demonstrate a correlation between operative systemic inflammation and cancer. Some biomarkers representing the degree of systemic inflammation, such as the Glasgow prognostic score, neutrophil lymphocyte ratio (NLR), and platelet lymphocyte ratio (PLR), have been shown to have prognostic value in many kinds of cancer patients [5-8]. However, these results only focus on the effects of preoperative cancer or inflammation, regardless of etiology of the inflammation. Until now, there is only a few research regarding postoperative systemic inflammation on cancer prognosis. In this study, we examine not only the effect of the preoperative inflammatory state on cancer, but also the effects of early postoperative systemic inflammation on cancer prognosis, based on the previous animal study results.
Materials and Methods
1. Patient settings
Six hundred thirty-nine consecutive patients underwent surgery for colorectal cancer at Yeouido St. Mary's Hospital, The Catholic University of Korea from January 2006 to December 2009. Patients were excluded if they had colorectal cancer other than adenocarcinoma and carcinoma in situ. However, patients who had preoperative radiation therapy and those who were not able to undergo curative resection were included. Finally, six hundred patients were included. The clinicopathologic data collected from a prospectively maintained database were analyzed.
Routine laboratory measurements, including white blood cell (WBC) count, neutrophil count, lymphocyte count, monocyte count, and platelet count, were performed preoperatively, daily until day four postoperatively, and subsequently every two days. We did not consider preoperative infection and inflammatory condition and postoperative infection and inflammatory condition. Patients were divided into three groups based on the days spent on the leukocyte count to drop below 10,000/mm3 after surgery (DSNLC; group I, 0-1 days; group II, 2-3 days; group III, ≥4 days). For a comparative analysis with the results of the other study, grouping of the variables, such as the WBC count, neutrophil count, lymphocyte count, monocyte count, and platelet count, was carried out using standard thresholds [4]. "Right side colon" was defined as both the right colon and the transverse colon. "Left side colon" was defined as both the left colon and the recto-sigmoid colon above the peritoneal reflection. Staging evaluation was carried out according to the guidelines of American Joint Committee on Cancer, sixth edition [9]. This study was approved by the Institutional Review Board of the College of Medicine (SC11TISI0080).
2. Statistical analysis
The relationship between the number of days required for the leukocyte count to drop below 10,000/mm3 after surgery (group I, 0-1 day; group II, 2-3 days; group III, ≥4 days) and clinicopathologic factors was assessed using the chi-squared and Fisher's exact tests.
The overall duration of survival was calculated from the date of surgery until the date of death. The duration of cancer-free survival was calculated from the date of surgery until the date of detection of disease recurrence, defined using clinical, radiographic or pathological findings. Overall and cancer-free survival rates were calculated using the Kaplan-Meier method, and differences among curves were tested using the log-rank test. The univariate prognostic significance of variables was determined using the Cox proportional hazard model. Variables that were significantly related to the survival rate in a univariate analysis were subsequently included in a multivariate analysis employing the Cox multiple regression model. This method finds the 'best' variables (p<0.05) for which the effects on prognosis are not only significant, but also independent when correlated with the other factors included in the model. All statistical tests were two-tailed and the significance level was set at 0.05. Statistical analyses were performed using the SPSS ver. 14.0 (SPSS Inc., Chicago, IL).
Results
1. Patient characteristics
The male-to-female ratio of patients was 1.48:1, and the mean age was 62.3±11.5 years. Three hundred fifty patients (58.2%) were under 65 years old, and 250 patients (41.7%) were over 65 years old. The follow-up period ranged from 1 to 72 months (mean, 27.4±18.2 months). Tumors were localized to the right side colon in 156 patients (26.0%), the left side colon in 250 patients (41.7%), the rectum in 184 patients (30.7%) and multiple areas in 10 patients (1.7%). There were no differences between the groups with regards to complication of cancer, which was defined by perforating or obstructing colorectal lesions. Among the rectal cancer patients, 27 patients underwent preoperative radiotherapy. Laparoscopic treatment was performed in 69 patients (11.5%), and conventional treatment was performed in 531 patients (88.5%). Five hundred thirty-one patients (88.5%) underwent R0 resection and 69 patients (11.5%) underwent R1 resection. We observed stage I disease in 140 patients (23.3%), stage II disease in 178 patients (29.7%), stage III0 disease in 208 patients (34.7%), and stage IV disease in 52 patients (8.7%). There were no differences in the characteristics of patients between the three groups, other than neoadjuvant RT and surgical approach methods (p=0.003 and p=0.005, respectively) (Table 1).
2. Clinicopathologic features related to systemic inflammatory response
In total, 548 patients (91.3%) had preoperative WBC counts under 10,000/mm3 and 52 patients (8.7%) had increased preoperative WBC counts (over 10,000/mm3). Postoperative WBC counts exceeded 10,000/mm3 in 417 patients (69.5%), including 74 cases (45.8%) in group I, 198 cases (33.0%) in group II, and 127 cases (21.2%) in group III. Preoperative WBC counts correlated significantly with disease stage (p=0.047). In particular, increased preoperative WBC counts were noted in patients at stages II and III (data not shown). However, neither postoperative WBC counts nor the number of days required for the leukocyte count to fall below 10,000/mm3 correlated with disease stage (p=0.394 and p=0.402, respectively). Patients who received preoperative radiotherapy and patients who had laparoscopic surgery had statistically significant shorter WBC normalization periods compared to patients without preoperative radiotherapy and with conventional surgery. The results did not show a significant difference in the postoperative WBC normalization period for the combined resection of other organs (p=0.329). The duration of postoperative hospital stay, according to the WBC normalization period, was as follows: group I, 12±7.1 days (median, 11.0 days); group II, 12.3±7.3 days (median, 10.0 days); group III, 14.8±7.9 days (median, 12.0 days). Groups I and II showed a significant difference from group III. However, there were no significant differences, between groups I and II.
There are significant correlations between the groups with regards to preoperative neutrophil count (p<0.001), lymphocyte count (p=0.001), monocyte count (p<0.001), and platelet count (p=0.001), but no correlation between preoperative NLR (p=0.47) (Table 1).
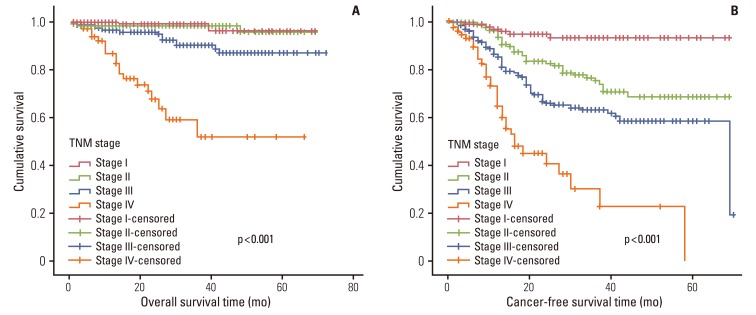
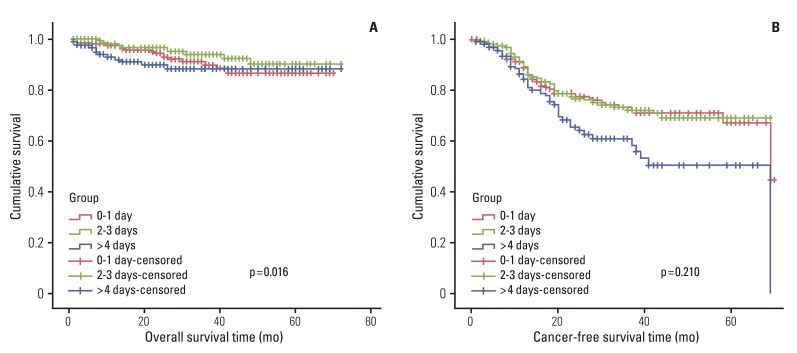
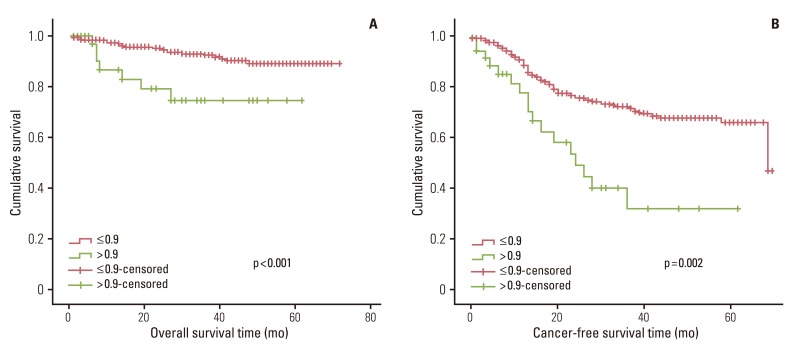
1) Survival in relation to clinicopatholigicfactors and systemic inflammatory response
In univariate survival analyses, tumor, node, metastasis (TNM) stage and monocyte count were significantly associated with cancer-free survival (p<0.01 for both parameters). In addition, cancer-free survival outcomes were significantly worse in patients who required more than four days for the normalization of WBC count (hazard ratio [HR], 1.652; 95% confidence interval [CI], 1.105 to 2.468; p=0.014). A TNM stage greater than II and NLR is significantly associated with the duration of overall survival (p<0.050 and p=0.045, respectively), but no correlation was observed between DSNLC and duration of overall survival (Table 2). Cancer-free and overall survival curves are shown according to the stage, time for leukocyte count to decline below 10,000/mm3 and monocyte count in Figs. 1, 2, and 3, respectively. No correlation was revealed between the elevated preoperative and postoperative WBC counts (≥10×109) and cancer-free or overall survival. In a multivariate analysis of these significant variables (except curative resection), TNM stage (stage II: HR, 3.545; 95% CI, 1.571 to 7.997; p=0.002; stage III: HR, 6.115; 95% CI, 2.818 to 13.447; p<0.001; stage IV: HR, 15.928; 95% CI, 6.974 to 36.378; p<0.001), an interval longer than four days for normalization of WBC counts (HR, 1.509; 95% CI, 0.999 to 2.278; p=0.050), and monocyte count (HR, 2.133; 95% CI, 1.262 to 3.605; p=0.005) were independently associated with cancer-free survival (Table 3). The overall survival was only associated with stage.
Discussion
There is evidence showing a linkage between cancer and inflammation. First, inflammatory diseases increase the risk of developing many types of cancer and inflammatory cells. Second, chemokines and cytokines are present in the microenvironment of all tumors in experimental animal models and in humans from the earliest stages of development [2]. As mentioned above, our experimental mouse model creates an inflammatory reaction in the peritoneum and confers an increased metastatic burden at the wound site compared with the undamaged normal peritoneal tissues [4]. Furthermore, this model demonstrates an abundance of pro-inflammatory cells at the wound site, which likely induces mesothelial cells in the peritoneum. Consequently, an increase in the mesothelial cells and other inflammatory cells constitute a pro-metastatic microenvironment in the normal peritoneum. In addition, in the early stages of wound healing, wound metastasis occurs. For wounds with continued inflammation, we confirmed that wound metastasis occurs even after seven days of wound healing.
The Glasgow Prognostic Score (GPS), which includes only serum C-reactive protein and serum albumin, has a prognostic value independent of stage in patients with advanced or primary operable cancer [5,6,10,11]. In addition, the NLR and PLR have been shown to have an independent prognostic value in a variety of cancers [5-8]. These studies demonstrate that the prognostic values of NLR or PLR are independent of tumor stage, conventional scoring systems and treatment modalities. Furthermore, studies conducted by Leitch et al. [6] show that monocyte count and GPS are independently associated with cancer-specific survival. In this study, monocyte count was significantly associated with cancer-free survival in a univariate survival analysis. NLR was significantly associated with overall survival (p=0.045). Postoperative WBC count (≥10×109) did not reveal any correlation with cancer-free survival or overall survival.
Most studies mentioned above examined the effects of the preoperative inflammatory condition. However, the research presented in this report does not focus on one point of time, but rather on a certain period of an inflammatory condition that will have a great effect on the metastatic microenvironment. This idea was brought on by the fact that MMP-9 increases in the first three days postoperatively during wound healing and that the early postoperative changes affect peritoneal metastasis, as observed in the author's prior animal model experiment study [4-12]. Thus, in the current study, regardless of the preoperative and postoperative inflammatory status, the groups were classified by the normalization of WBC count. The period in which the metastatic microenvironment is exposed to inflammation was defined as the period until the WBC count is normalized. The results did not show significant differences between the groups, the cancer location, the extent of curative resection, or stage. The increase in postoperative WBC cell count did not differ according to the stage. These results demonstrate that the differences in surgical methods according to stage did not affect the postoperative WBC count or DSNLC.
In a univariate analysis, the presence of increased WBCs in the preoperative stages did not affect the duration of disease-free survival or overall survival. On the other hand, sustained inflammation over four days did affect disease-free survival and overall survival. We can analyze that this sustained inflammation can also have a systematic effect, like the result of three day inflammatory cell increase of wound healing process having an effect on peritoneal metastasis. Moreover, DSNLC was independently associated with cancer-free survival in a multivariate survival analysis.
Other studies have shown that an elevated GPS may reflect an altered innate immune response, as GPS was also associated with increased numbers of neutrophils and monocytes. In addition, these results were strongly associated with poorer cancer-specific survival. This may suggest that activation of innate immunity, rather than down-regulation of acquired immunity, is the most important factor in determining a poor outcome in patients with colorectal cancer [6]. One study also revealed that an early increase in WBCs during surgery correlates with an increased innate immune response. In addition, an increase in WBCs is a better prognostic parameter than an increase in monocytes or NLR. In this study, an early operative increase in WBC correlates with increased innate immunity, and this increase is predictive of a poorer outcome for colorectal cancer more so than monocyte or NLR increase, which only shows an increase in innate immunity at one point of time.
Preoperative WBC count shows a correlation with an increase of cancer stage, with the exception of stage IV. It has a correlation with the degree of cancer. In stage IV, since neoadjuvant chemotherapy can be performed, an increase in WBCs may be lower than in stages II and III. In addition, patients receiving preoperative radiation show statistically significant shorter periods for normalization of WBC counts compared to patients not receiving radiation therapy. Generally, in radiation therapy, numerous inflammatory cells are recruited into the irradiation field. Gross inflammatory scores and microscopic inflammatory scores are significantly higher at the colonic anastomotic line after preoperative irradiation [13,14]. However, in colorectal cancer, the systemic effect of radiotherapy may not be substantial, and instead, the accompanying chemotherapy may be the reason for a shorter period of normalization of WBC count. In addition, there is a shorter WBC count normalization period following laparoscopic surgery; this is an advantage of minimally invasive surgery. En bloc resection with radical lymphadenectomy is performed by laparoscopic surgery in our center in the same manner of open surgery. Since there is no difference in operative extent between surgical methods, the shorter WBC count normalization period and decreased persistence of inflammation is attributable to laparoscopic surgery. However, there is no difference in the duration of disease-free survival between laparoscopic and open surgery. Postoperative increase in WBC count has many contributory factors, such as cancer cell itself, surgical complications, radiation therapy, chemotherapy and surgical methods. Whatever the causative factor, the increase in postoperative WBCs affects the metastatic microenvironment and eventually contributes to cancer recurrences. Postoperative WBC count increase did not show any correlation with cancer stage. WBC count decrease was observed in cases that had preoperative radiation or chemotherapy. For laparoscopic surgery, more research is necessary as there are relatively few cases; thus other factors, such as selection bias, can influence the results. Complicated cancer was defined by perforating or obstructing colorectal lesions shows a correlation with an increase of preoperative WBC count (p=0.004), but there were no differences between the groups (p=0.694, data not shown). This fact shows that complicated colorectal cancer was not correlative with the normalization of WBC count unless the lesion persists.
In addition, the WBC count normalization period after surgery did not correlate with the presence of combined resection, but rather correlated with the duration of postoperative hospital stay. In this study, three factors (surgical approach method, preoperative radiation therapy and postoperative hospital stay) were found to be associated with the difference in time of the period of normalization of WBC count. The other factors were not associated with the differences. Postoperative complications, factors in the preoperative period or factors that can affect the cancer itself may also be considered. Among these additional factors, unfortunately, postoperative complications were not recorded prospectively. Postoperative complications were omitted as a factor, leading to inaccuracy in the retrospective study, and this represents a shortcoming of this study. Taking into account the postoperative hospital stay, it can be inferred that postoperative complications are likely to have a correlation.
Conclusion
In summary, postoperative early inflammatory phase and preoperative monocyte count correlate with the poor prognosis of colon cancer even after the calibration of stage. Thus, we can conclude that preoperativeand postoperative inflammatory response and period unfavorably affect the metastatic microenvironment.