AbstractPurposeThis phase I trial evaluated the question of whether the standard starting dose of axitinib could be administered in combination with therapeutic doses of cisplatin/capecitabine in patients with previously untreated advanced gastric cancer, and assessed overall safety, pharmacokinetics, and preliminary antitumor activity of this combination.
Materials and MethodsPatients in dose level (DL) 1 received axitinib 5 mg twice a day (days 1 to 21) with cisplatin 80 mg/m2 (day 1) and capecitabine 1,000 mg/m2 twice a day (days 1 to 14) in 21-day cycles. Maximum tolerated dose (MTD) was the highest dose at which ≤ 30% of the first 12 patients experienced a dose-limiting toxicity (DLT) during cycle 1. Ten additional patients were enrolled and treated at the MTD in order to obtain additional safety and pharmacokinetic data.
ResultsThree DLTs occurred during cycle 1 in three (25%) of the first 12 patients: ruptured abdominal aortic aneurysm, acute renal failure, and > 5 consecutive days of missed axitinib due to thrombocytopenia. DL1 was established as the MTD, since higher DL cohorts were not planned. Common grade 3/4 non-hematologic adverse events in 22 patients treated at DL1 included hypertension (36.4%) and decreased appetite and stomatitis (18.2% each). Cisplatin/capecitabine slightly increased axitinib exposure; axitinib decreased capecitabine and 5-fluorouracil exposure. Eight patients (36.4%) each had partial response or stable disease. Median response duration was 9.1 months; median progression-free survival was 3.8 months.
IntroductionGastric cancer is the fourth most common malignancy and the second leading cause of cancer death worldwide [1]. Cases of gastric cancer in Eastern Asia account for half of the world total, and the highest estimated mortality rates are reported in those countries. For advanced gastric cancer, first-line therapy with combination chemotherapy results in improved overall survival (OS) compared with best supportive care or single-agent chemotherapy. Treatment guidelines [2], therefore, recommend first-line combination regimens incorporating a platinum agent and a fluoropyrimidine. There is controversy regarding the benefit of adding a third chemotherapeutic agent.
Different targets contributing to the pathogenesis of gastric cancer have been explored in attempts to improve outcomes. Human epidermal growth factor receptor 2 (HER2) is an important target in gastric cancer. The combination of trastuzumab, a HER2-targeted agent, with cisplatin and a fluoropyrimidine significantly improved median OS compared with chemotherapy alone as first-line therapy in patients with HER2-postive advanced gastric cancer [3]. In addition, expression of vascular endothelial growth factor (VEGF) is more likely to occur in gastric tumors than in non-cancerous tissue, and VEGF expression is associated with worse survival in patients with gastric cancer [4,5]. Several VEGF-targeted agents (bevacizumab [6], sorafenib [7], sunitinib [8-10], and ramucirumab [11]) have been evaluated in patients with advanced gastric cancer. In a phase III randomized trial, ramucirumab plus best supportive care improved OS compared with best supportive care alone as second-line therapy in patients with metastatic gastric cancer [11].
Axitinib is a potent and selective second-generation inhibitor of VEGF receptors 1, 2, and 3 [12]. It is approved in the United States, European Union, and other countries for treatment of advanced renal cell carcinoma after failure of one prior systemic therapy, based on the phase III AXIS trial, which demonstrated that axitinib significantly improved progression-free survival (PFS) compared with sorafenib as second-line therapy [13]. Phase II trials have evaluated the antitumor activity of axitinib as a single agent in thyroid cancer [14], non-small cell lung cancer [15], and melanoma [16], and in combination with chemotherapy in pancreatic [17], breast [18], colorectal cancer [19], and other indication.
This phase I trial was designed to evaluate the tolerability of axitinib administered at the starting dose of 5 mg twice a day in combination with therapeutic doses of cisplatin/capecitabine in patients with advanced gastric cancer.
Materials and Methods1. Study designThis open-label phase I trial evaluating axitinib in combination with cisplatin/capecitabine in patients with previously untreated advanced gastric cancer was conducted at three sites in Korea and two sites in Japan. The primary objective of this trial was to assess the tolerability of axitinib administered at the standard starting dose in combination with cisplatin/capecitabine, and to determine the maximum tolerated dose (MTD) of the combination regimen by evaluating first-cycle dose-limiting toxicities (DLTs) and overall safety. The secondary objectives were to evaluate the plasma pharmacokinetics (PKs) of axitinib, cisplatin, and capecitabine and its metabolite 5-fluorouracil (5-FU) when administered at the MTD, and to assess preliminary antitumor activity of the combination.
The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guideline on Good Clinical Practice, the study protocol, and applicable local regulatory requirements and laws. The study protocol, amendments, and informed consent forms were approved by an Institutional Review Board/Independent Ethics Committee. All participants provided written informed consent. The trial is registered on ClinicalTrials.gov (NCT00842244).
2. PatientsPatients aged ≥ 20 years at Japanese sites or ≥ 18 years at Korean sites with stage IV gastric or gastroesophageal junction adenocarcinoma (type II or III tumors) for which curative intent was not possible, and at least one unidimensionally measureable lesion using a modified Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.0 were eligible. Patients were required to have Eastern Cooperative Oncology Group performance status of 0 or 1; adequate bone marrow, renal, and hepatic function; and blood pressure (BP) ≤ 130/80 mm Hg. Antihypertensive medications were permitted.
Patients who had undergone radiation therapy or surgery within 4 weeks prior to study entry or prior treatment with a systemic anticancer agent for advanced gastric cancer were excluded. Palliative radiation therapy to non-target lesions within 2 weeks prior to study entry was allowed. Patients with disease progression while on prior adjuvant treatment or progression-free interval < 6 months after completion of adjuvant therapy were not permitted. Because the trastuzumab trial [3] results were unknown when this trial was designed, HER2 status was not assessed.
3. Study treatmentsPatients in dose level (DL) 1 received cisplatin 80 mg/m2 intravenously over 2 hours on day 1 and capecitabine 1,000 mg/m2 twice a day orally within 30 minutes after a meal on days 1 to 14 of each 21-day cycle. Axitinib was taken orally with food at a starting dose of 5 mg twice a day on days 1 to 21. On day 1, a 5-hydroxytryptamine3-receptor antagonist and dexamethasone were administered before cisplatin, with vigorous hydration according to institutional practices.
In cycle 1, patients in the PK expansion subgroup received lead-in dosing with axitinib beginning on day 3 and continuing through day 18, with administration of cisplatin on day 1 and capecitabine on days 1 to 14. On day 19 of cycle 1, axitinib was temporarily stopped for 3 days before cycle 2. In cycle 2, cisplatin was administered on day 1 and capecitabine on days 1 to 14, with axitinib restarted on day 2 and continued without any planned interruptions thereafter (Fig. 1). For cycle 3 and beyond, all three drugs were started on day 1.
If more than one of the first six patients experienced a DLT (Table 1) during cycle 1, patients would have been enrolled in DL –1: axitinib 3 mg twice a day on days 1-21 or cisplatin 60 mg/m2 on day 1 or capecitabine 800 mg/m2 twice a day on days 1-14 of each 21-day cycle. The actual drug dose reductions were to be determined by the toxicity profile observed. Cohorts at higher DLs were not planned.
Intrapatient dose modifications for axitinib and chemotherapy were permitted based on tolerability. Investigators, in discussion with the sponsor, had the discretion to discontinue, delay, or modify the dosages of the drugs depending on the severity and timing of adverse events (AEs). Axitinib-related hypertension (BP > 150/100 mm Hg) was managed by increasing the dose of existing or adding new antihypertensive medication. If the patient was already on maximal antihypertensive therapy, axitinib was to be reduced to the next lower dose (i.e., 3 or 2 mg twice a day). If the patient developed recurrent BP > 150/100 mm Hg, axitinib was to be reduced to the next lower dose; reductions < 2 mg twice a day required discussion with the sponsor. If BP was > 160/105 mm Hg, axitinib was to be stopped and then restarted at the next lower dose when BP returned to < 150/100 mm Hg. Once the dose was reduced for axitinib-related toxicity, it was not generally re-escalated.
Patients were treated until disease progression, intolerable toxicity, withdrawal of consent, or removal from the study by the investigator. Patients requiring the use of strong cytochrome P450 (CYP) 3A4/5 or CYP1A2 inducers or strong CYP3A4/5 inhibitors were not eligible for enrollment; however, if these drugs were required during the study, they were allowed and adjustment of axitinib dosage was to be considered.
4. AssessmentsTo determine the MTD, initially three patients were to be treated at DL1. If no more than one DLT (Table 1) was observed during cycle 1, another three patients were to be enrolled at DL1 to complete the enrollment of six patients into the cohort. If cycle 1 toxicities at DL1 exceed the MTD, patients would be enrolled in DL –1. If no more than one of the six patients experienced a DLT during cycle 1, another six patients were to be entered at DL1. The MTD was the highest dose at which ≤ 30% of the 12 patients experienced a DLT during cycle 1. If DL1 did not exceed the MTD for the combination, this would be declared the MTD as higher dose level cohorts were not planned in this study. An additional 10 patients were to be enrolled in an expansion cohort at the MTD for collection of further safety, PK, and antitumor activity data.
Safety was based on the incidence and severity (graded by Common Terminology Criteria for Adverse Events ver. 3.0) of AEs, and assessment of laboratory tests, home BP monitoring, and physical examinations. Tumors were assessed radiologically using a modified RECIST ver. 1.0 at screening, every 6 weeks, and whenever disease progression was suspected.
PK sampling and analysis for axitinib, cisplatin, and capecitabine and its metabolite 5-FU in plasma were performed for 10 patients in the MTD expansion cohort from whom complete data were available. Standard plasma PK parameters, including maximum observed plasma concentration (Cmax), time at which Cmax was observed (Tmax), area under the curve (AUC) from time 0 extrapolated to infinite time (AUCinf), AUC from time 0 to 24 hours (AUC24), terminal half-life (t1/2), clearance (CL) or apparent oral clearance (CL/F), and volume of distribution during the elimination phase (Vz) or apparent volume of distribution during the elimination phase (Vz/F), were estimated using the validated eNCA ver. 2.2.1 (Pfizer Inc., New York, NY; proprietary software for non-compartmental parameter estimation).
The study design allowed for evaluation in each patient of the PK of steady-state axitinib alone on cycle 1, day –1; cisplatin/capecitabine alone on cycle 2, day 1; and cisplatin/capecitabine plus steady-state axitinib on cycle 1, day 1 (Fig. 1). Axitinib PK samples were collected prior to and 1, 2, 3, 4, 6, and 8 hours after axitinib dosing. Capecitabine PK samples were collected prior to and 0.25, 0.5, 1, 2, 3, 4, 6, and 8 hours after capecitabine dosing. Cisplatin PK samples were collected prior to the start of cisplatin infusion, 0.5 and 1 hours (during infusion), 2 hours (end of infusion), and 1, 2, 4, and 6 hours after the end of infusion. Cisplatin PK samples were drawn from the arm not receiving chemotherapy.
Plasma concentrations of axitinib were measured using a validated, sensitive, and specific high-performance liquid chromatography with tandem mass spectrometric (HPLC/MS/MS) detection method (Charles River Laboratories Preclinical Services, Shrewsbury, MA). The lower limit of quantification (LLOQ) for axitinib was 0.500 ng/mL. Platinum (derived from cisplatin) concentrations in plasma and plasma ultrafiltrate (PUF) were analyzed (Covance Laboratories, Madison, WI) using validated, sensitive, and specific inductively coupled plasma mass spectrometric methods. The LLOQ for platinum was 2.00 ng/mL in plasma and 1.00 ng/mL in PUF. Analysis of plasma samples (WuXi AppTec, Shanghai, China) for concentrations of capecitabine and its metabolite 5-FU was performed using a validated, sensitive, and specific HPLC/MS/MS method. The LLOQ was 20.0 ng/mL for capecitabine and 5.00 ng/mL for 5-FU.
5. Statistical analysisThe study sample size was dependent on the observed safety profile of axitinib plus chemotherapy, which determined the number of patients per DL. Analysis of efficacy and safety was performed for all patients who received at least one dose of study medication. Descriptive statistics (mean, median, standard deviation, and range for continuous data; percentage for categorical data; and 95% confidence interval [CI], if applicable) were used to summarize patient characteristics, treatment administration/compliance, antitumor activity, safety, and PK parameters. The Kaplan-Meier method was used to estimate the duration of response (time from first demonstration of partial or complete response to documented disease progression) and PFS (time from start date to date of first documentation of progression or death due to any cause). The study was not powered for detection of differences in PK parameters for capecitabine, cisplatin, or axitinib alone or in combination. All analyses were performed based on final data following database closure, with a data cutoff date of October 17, 2012.
Results1. Patients and treatmentA total of 22 patients were enrolled in DL1 between April 2009 and April 2010. Patient demographic and baseline characteristics are summarized in Table 2. All patients discontinued the study because of either disease progression (n=16; leading to death in two patients during the study period), death (n=1; due to ruptured abdominal aortic aneurysm), AEs (n=2), refusal of further follow-up or treatment (n=1 each), or enrollment in an ongoing extension study (n=1; NCT00828919). The two AEs leading to study discontinuation were acute renal failure and gastrointestinal perforation.
Patients started a median of four cycles of combination therapy (range, 1 to 31 for axitinib, 1 to 10 for cisplatin, and 1 to 31 for capecitabine). Dose delays for axitinib, cisplatin, or capecitabine occurred in 15 patients (68.2%). Dose reductions for axitinib, cisplatin, and capecitabine were required in 19 patients (86.4%), 10 (45.5%), and 17 (77.3%), respectively. Median (range) relative dose intensity (percent of actual/intended dose intensity) was 70.1% (33.3% to 95.2 %) for axitinib, 76.5% (42.1% to 103.1%) for cisplatin, and 67.5% (38.7% to 93.9%) for capecitabine.
2. DLTs and MTDThree DLTs occurred during cycle 1 in three (25%) of the first 12 patients treated at DL1. In the first cohort of six patients, one patient had a DLT of ruptured abdominal aortic aneurysm (grade 5), which was considered to be related to all three study drugs. In the second cohort of six patients, one patient experienced a DLT of acute renal failure (grade 3) lasting 21 days, which was considered to be related to axitinib and cisplatin, and another patient missed > 5 consecutive days of axitinib doses due to thrombocytopenia (grade 3), which was considered to be related to all three study drugs. Per the definition of MTD in this study, axitinib 5 mg twice a day on days 1-21 in combination with cisplatin 80 mg/m2 on day 1 and capecitabine 1,000 mg/m2 twice a day on days 1-14 of each 21-day cycle was established as the MTD. In addition, gastrointestinal perforation (grade 3), which was considered to be related to all three study drugs, was reported as a DLT in one of the 10 patients in the MTD expansion cohort.
3. Adverse eventsThe most frequently reported all-causality, all-grade, non-hematologic AEs (Table 3) included decreased appetite (n=20, 90.9%), nausea and fatigue (n=17, 77.3% each), and hypertension and stomatitis (n=16, 72.7% each). The most common all-causality, grade 3/4, non-hematologic AEs were hypertension (n=8, 36.4%) and decreased appetite and stomatitis (n=4, 18.2% each). During the study, a total of 12 patients experienced 18 serious AEs: thrombosis, headache, hypoesthesia, enterocolitis, diarrhea, pneumonia, acute prerenal failure, ruptured abdominal aortic aneurysm, bradycardia, hypertension, nausea, vomiting, gastrointestinal hemorrhage, acute renal failure, decreased appetite, and gastrointestinal perforation (n=1 each), and disease progression leading to death (n=2). All-causality laboratory abnormalities are summarized in Table 3. The most common grade 3/4 laboratory abnormalities included neutropenia (n=8, 36.4%) and thrombocytopenia (n=4, 18.2%).
4. PharmacokineticsPK parameters for axitinib at steady-state, cisplatin, and capecitabine and its metabolite 5-FU in the absence and presence of each other are summarized in Table 4, and the respective plasma concentration-time profiles are shown in Fig. 2. Administration of cisplatin/capecitabine resulted in slightly increased axitinib plasma exposure, as indicated by a higher AUC24 and Cmax. Axitinib did not appear to alter cisplatin (platinum in PUF) exposure, but it did decrease plasma exposure of capecitabine and 5-FU, as suggested by lower AUC24 and Cmax (Table 4).
5. Antitumor activityThe overall investigator-assessed objective response rate (ORR) was 36.4% (95% CI, 17.2% to 59.3%). Eight patients (36.4%) each had partial response or stable disease (≥ 6 weeks). Maximum percent change in tumor size from baseline in target lesions is shown in Fig. 3. Median duration of response in patients with partial response was 9.1 months (95% CI, 6.4 to 20.2 months); median PFS was 3.8 months (95% CI, 2.9 to 9.4 months).
DiscussionCisplatin/5-FU has long been a standard first-line treatment option for patients with advanced gastric cancer; cisplatin/capecitabine has also become an acceptable alternative. We evaluated axitinib in combination with cisplatin/capecitabine as first-line therapy in patients with advanced gastric cancer. Because DLs higher than DL1 were not planned in this trial, all patients were started at DL1 consisting of axitinib 5 mg twice a day on days 1 to 21 in combination with cisplatin 80 mg/m2 on day 1 and capecitabine 1,000 mg/m2 twice a day on days 1 to 14 of each 21-day cycle. Three DLTs were observed during cycle 1 in three (25%) of the first 12 patients (ruptured abdominal aortic aneurysm, acute renal failure, and > 5 consecutive days of missed axitinib doses due to thrombocytopenia). As per the trial’s definition, we established that DL1 was the MTD. One additional event (gastrointestinal perforation) was reported as a DLT in the MTD expansion cohort. The combination appeared to be generally tolerated with a manageable safety profile.
Compared with cytotoxic chemotherapy, most molecularly targeted agents, such as axitinib [13], are relatively well tolerated, with most AEs reported as grade 1 or 2. However, chronic administration of these agents with relatively short-term or no holidays may result in new challenges in terms of cumulative toxicity [20]. The combination of molecularly targeted agents with chemotherapy may augment this fundamental issue associated with chronic administration. Although potentially manageable during the first cycle, when chronic, overlapping toxicities and delayed recovery from chemotherapy-related toxicity, as a result of molecularly targeted agents chronically altering physiologic cellsignaling pathways in normal tissue can become unacceptable. Consequently, this may require drug dose reductions or delays. We determined tolerable doses to be the standard starting dose of axitinib and therapeutic doses of cisplatin/capecitabine, but median relative dose intensity was only ~70% for both axitinib and chemotherapy. The low median relative dose intensities for axitinib, cisplatin, and capecitabine reflect the number of patients experiencing dose delays or reductions due to AEs for all three drugs.
Given the importance of maintaining the dose intensity of chemotherapy to preserve efficacy, a new paradigm is urgently needed for determining the optimal dosing schedule in phase I studies of molecularly targeted agents combined with chemotherapy.
In terms of PK interaction, axitinib is primarily metabolized by CYP3A4/5, and to a lesser extent (< 10% each) by CYP1A2, CYP2C19, and uridine diphosphate glucuronosyltransferase 1A1 [21]. Cisplatin does not undergo substantial enzymatic biotransformation in the liver and is mainly eliminated through renal excretion [22,23]; hence, induction or inhibition of CYP450 activity by concomitant medications is not likely to affect platinum CL. Capecitabine, a pro-drug, undergoes a three-step metabolic process to its active component 5-FU [24]. Overall, interactions between axitinib and cisplatin or capecitabine are considered unlikely as axitinib is metabolized via pathways that are distinct from those for cisplatin and capecitabine.
Our data indicate that coadministration of axitinib did not overtly affect the PK of cisplatin. This is consistent with expectations, based on knowledge of the disposition of the two drugs. Although the sample size for PK evaluation was small in this study (n=6 for assessment of capecitabine and n=3 for 5-FU plasma exposure), there is a suggestion of decreased plasma exposure for capecitabine and its metabolite 5-FU in the presence of axitinib. The mechanism by which axitinib may decrease capecitabine or 5-FU exposure is not clear. Results also indicate that axitinib plasma exposure was increased in the presence of cisplatin/capecitabine. Although the current study was not statistically powered for PK evaluations, the ~30% higher axitinib exposure in the presence of cisplatin/capecitabine was within the typical intersubject variability in axitinib exposure (% coefficient of variation for axitinib AUC24=79% to 83%) observed in this study.
In patients with advanced gastric cancer, preliminary antitumor activity of axitinib with cisplatin/capecitabine included a 36.4% ORR and 3.8-month median PFS. Other VEGF-targeted agents have been evaluated in patients with advanced gastric cancer [6-11]. Sunitinib [10], sorafenib [7], and bevacizumab [6] have been combined with cisplatin/capecitabine. In a phase I trial, patients receiving sunitinib with cisplatin/capecitabine had a 5.5-month median PFS and 33% ORR [10]. In the phase I trial of sorafenib with cisplatin/capecitabine, patients had a 10-month median PFS and a 62.5% ORR [7].
The addition of bevacizumab 7.5 mg/kg to cisplatin 80 mg/m2 (day 1) plus capecitabine 1,000 mg/m2 twice a day (days 1-14) every 3 weeks in the phase III AVAGAST trial [6] improved median PFS (6.7 months vs. 5.3 months; hazard ratio, 0.80; 95% CI, 0.68 to 0.93; p=0.0037) and ORR (46.0% vs. 37.4%, p=0.0315) in patients with previously untreated advanced gastric cancer. However, the primary endpoint of OS improvement was not achieved.
This small, exploratory phase I study has limitations, and definitive conclusions cannot be drawn regarding the synergistic effect of this combination on antitumor activity. In addition, we currently lack definitive biomarkers for use in predicting which patients will experience long durations of response. Because standard prescribing doses for individual drugs were used in DL1 and higher DL cohorts were not planned in this study, we know that the doses evaluated were tolerable but we may not know the true MTD. Larger prospective studies may be needed in order to further investigate the safety and antitumor activity of axitinib in combination with cisplatin and capecitabine in patients with advanced gastric cancer.
ConclusionIn conclusion, in patients with advanced gastric cancer, standard doses of axitinib (5 mg twice a day continuously) plus therapeutic doses of cisplatin (80 mg/m2 on day 1) and capecitabine (1,000 mg/m2 twice a day on days 1-14) could be administered in 21-day cycles in combination. The AEs encountered with this regimen were manageable.
Conflicts of InterestYing Chen, Liqiang Yang, and Olga Valota are Pfizer employees and hold Pfizer Inc. stock. Yung-Je Bang has performed consulting services for and received honoraria and research funding from Pfizer Inc. Do-Youn Oh, Toshihiko Doi, Kuniaki Shirao, Sook Ryun Park, and Keun-Wook Lee have no conflicts of interest to disclose. This study was sponsored by Pfizer Inc. Medical writing support was provided by Lilliam Poltorack, PharmD, and Joanna Bloom, PhD, of Engage Scientific Solutions, and was funded by Pfizer Inc. Fig. 1.Study design for patients in the pharmacokinetic (PK) subgroup. b.i.d., twice a day; D, day; C, cycle. a)Morning doses on day 1 were administered at the start of cisplatin infusion, b)1,000 mg/m2, c)80 mg/m2. 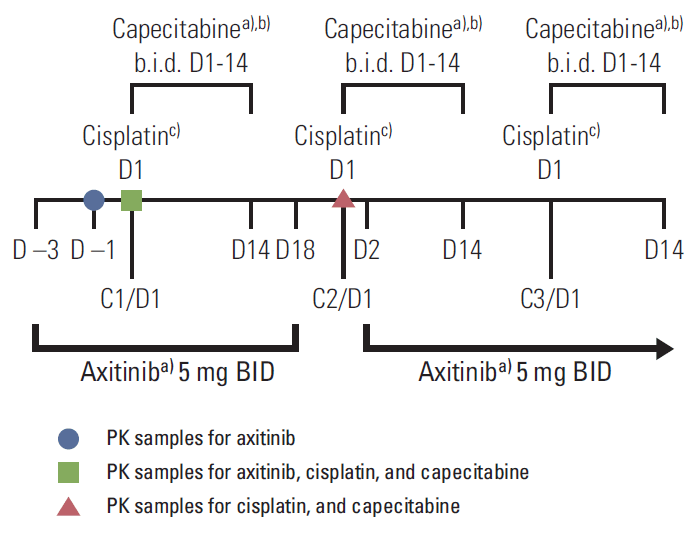
Fig. 2.Median plasma concentration-time profiles, semi-log scale, for steady-state axitinib (A), cisplatin (platinum in plasma ultrafiltrate [PUF]) (B), capecitabine (C), and 5-fluorouracil (5-FU) (D). The lower limit of quantification was 0.500 ng/mL for axitinib, 1.00 ng/mL for platinum in PUF, 20.0 ng/mL for capecitabine, and 5.00 ng/mL for 5-FU. Two patients were excluded from median concentration profile plots for platinum in PUF, capecitabine, and 5-FU because cycle 2 (C2), day 1 (D1) pharmacokinetic samples were not collected. 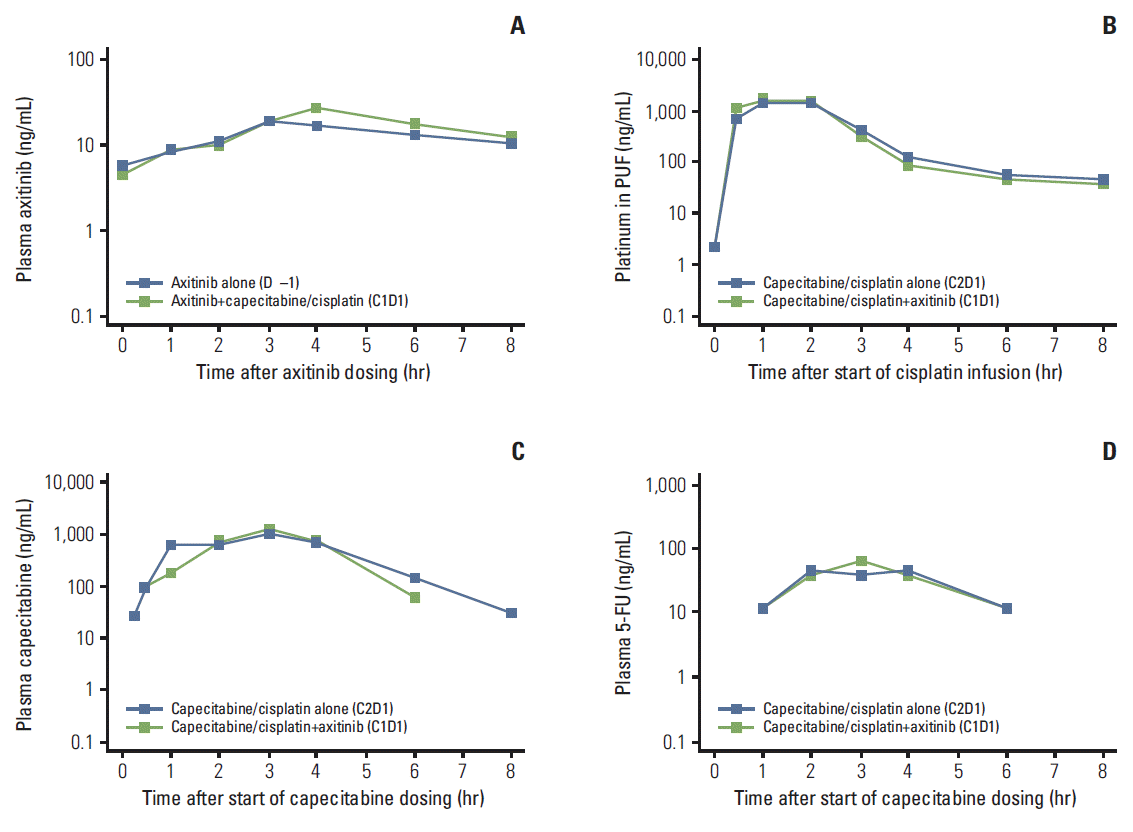
Fig. 3.Waterfall plot of tumor response for each patient (n=20) with at least one post-baseline scan. Two patients were not evaluable, and one patient had a maximum percent change from baseline of 0. 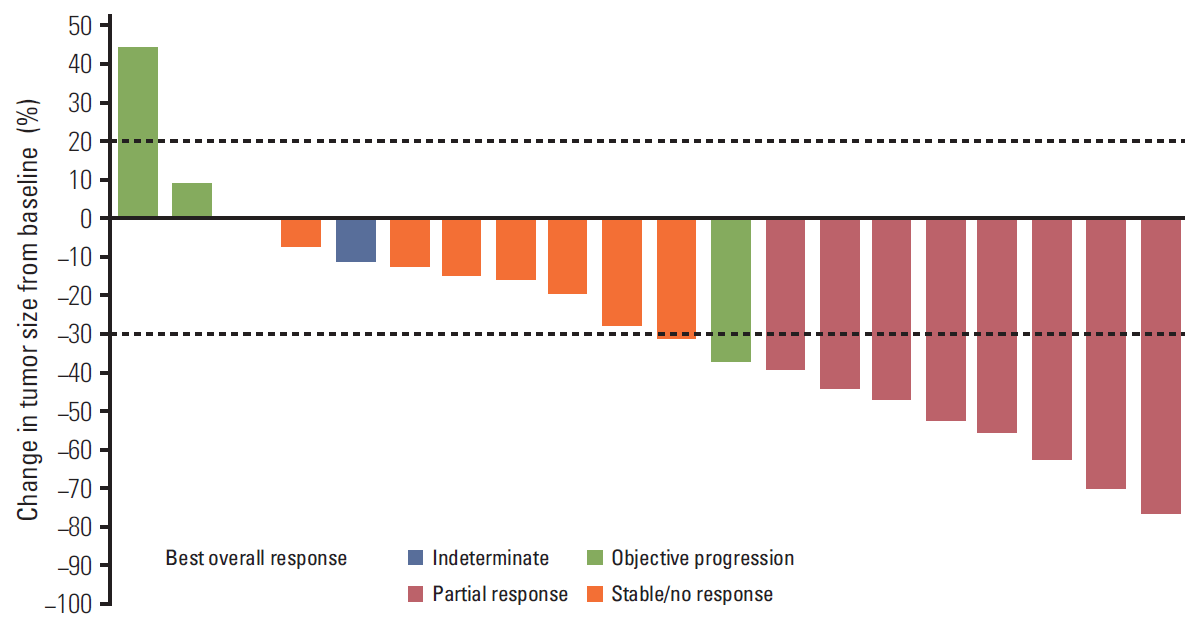
Table 1.Definition of dose-limiting toxicities during cycle 1
Table 2.Patient demographic and baseline characteristics (n=22)
Table 3.All-causality non-hematologic adverse events and laboratory abnormalities
Table 4.Summary of PK parameters for axitinib at steady-state, cisplatin, and capecitabine and its metabolite 5-FU in patients with advanced gastric cancer
PK, pharmacokinetic; 5-FU, 5-fluorouracil; CI, confidence interval; Cmax, maximum observed plasma concentration; AUC24, area under the curve from time 0 to 24 hours; Tmax, time at which Cmax was observed; CL/F, apparent oral clearance; Vz/F, apparent volume of distribution during elimination phase; t1/2, terminal half-life. b) AUC from time 0 extrapolated to infinite time (AUCinf) for total platinum in plasma ultrafiltrate, d) Systemic clearance (CL) and volume of distribution during elimination phase (Vz) for platinum in plasma ultrafiltrate, f) One patient was excluded from summary statistics for AUC24, CL/F, Vz/F, and t1/2 because of a non-estimable half-life, g) PK parameters are for platinum in plasma ultrafiltrate. Two patients were excluded from summary statistics for all PK parameters because PK samples from matching cycle 1 and cycle 2 were not completed. One patient was excluded from summary statistics for AUCinf, CL, Vz, and t1/2 due to non-estimable half-life, References1. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon: International Agency for Research on Cancer; 2010. [cited 2014 Mar 6]. Available from: http://globocan.iarc.fr/
2. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN Guidelines™) v2.2013 gastric cancer [Internet]. Fort Washington, PA: National Comprehensive Cancer Network; c2014. [cited 2014 Mar 6]. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp
3. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97.
4. Shi H, Xu JM, Hu NZ, Xie HJ. Prognostic significance of expression of cyclooxygenase-2 and vascular endothelial growth factor in human gastric carcinoma. World J Gastroenterol. 2003;9:1421–6.
5. Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, et al. Prognostic value of vascular endothelial growth factor expression in gastric carcinoma. Cancer. 1996;77:858–63.
6. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968–76.
7. Kim C, Lee JL, Choi YH, Kang BW, Ryu MH, Chang HM, et al. Phase I dose-finding study of sorafenib in combination with capecitabine and cisplatin as a first-line treatment in patients with advanced gastric cancer. Invest New Drugs. 2012;30:306–15.
8. Bang YJ, Kang YK, Kang WK, Boku N, Chung HC, Chen JS, et al. Phase II study of sunitinib as second-line treatment for advanced gastric cancer. Invest New Drugs. 2011;29:1449–58.
9. Moehler M, Mueller A, Hartmann JT, Ebert MP, Al-Batran SE, Reimer P, et al. An open-label, multicentre biomarker-oriented AIO phase II trial of sunitinib for patients with chemorefractory advanced gastric cancer. Eur J Cancer. 2011;47:1511–20.
10. Park SR, Lee KW, Oh DY, Park YI, Roh EJ, Khosravan R, et al. Sunitinib (SU) with cisplatin (P) and capecitabine (X) or oxaliplatin (O) and X (XELOX) in advanced gastric cancer (GC): a phase I, dose-finding study. In: the 35th Annual Congress of the European Society for Medical Oncology (ESMO); 2010 Oct 8-12; Milan, Italy. Viganello-Lugano: European Society for Medical Oncology; 2010.
11. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–9.
12. Hu-Lowe DD, Zou HY, Grazzini ML, Hallin ME, Wickman GR, Amundson K, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14:7272–83.
13. Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9.
14. Cohen EE, Rosen LS, Vokes EE, Kies MS, Forastiere AA, Worden FP, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–13.
15. Schiller JH, Larson T, Ou SH, Limentani S, Sandler A, Vokes E, et al. Efficacy and safety of axitinib in patients with advanced non-small-cell lung cancer: results from a phase II study. J Clin Oncol. 2009;27:3836–41.
16. Fruehauf J, Lutzky J, McDermott D, Brown CK, Meric JB, Rosbrook B, et al. Multicenter, phase II study of axitinib, a selective second-generation inhibitor of vascular endothelial growth factor receptors 1, 2, and 3, in patients with metastatic melanoma. Clin Cancer Res. 2011;17:7462–9.
17. Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, et al. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet. 2008;371:2101–8.
18. Rugo HS, Stopeck AT, Joy AA, Chan S, Verma S, Lluch A, et al. Randomized, placebo-controlled, double-blind, phase II study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J Clin Oncol. 2011;29:2459–65.
19. Infante JR, Reid TR, Cohn AL, Edenfield WJ, Cescon TP, Hamm JT, et al. Axitinib and/or bevacizumab with modified FOLFOX-6 as first-line therapy for metastatic colorectal cancer: a randomized phase 2 study. Cancer. 2013;119:2555–63.
20. Postel-Vinay S, Gomez-Roca C, Molife LR, Anghan B, Levy A, Judson I, et al. Phase I trials of molecularly targeted agents: should we pay more attention to late toxicities? J Clin Oncol. 2011;29:1728–35.
21. Chen Y, Tortorici MA, Garrett M, Hee B, Klamerus KJ, Pithavala YK. Clinical pharmacology of axitinib. Clin Pharmacokinet. 2013;52:713–25.
22. Vermorken JB, van der Vijgh WJ, Klein I, Hart AA, Gall HE, Pinedo HM. Pharmacokinetics of free and total platinum species after short-term infusion of cisplatin. Cancer Treat Rep. 1984;68:505–13.
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||