AbstractPurposeThis study was conducted in order to validate the radiosensitization effect of valproic acid, a biologically available histone deacetylase inhibitor, for fractionated radiation.
Materials and MethodsRadiosensitization effect of valproic acid was tested for the A549 cell line and U87MG cell line in vitro. Fractionated irradiation of 12 Gy in four fractions was administered on D2-5 with valproic acid, 150 mg/Kg, ip, bid for six consecutive days (D1-6) to A549 and U87MG tumors implanted in BALB/c-nude mice. A growth delay curve was formulated.
ResultsRadiosensitization effect of valproic acid was found for both cell lines; A549 at 1.5 mM and 3.0 mM concentration and U87MG at 3.0 mM concentration. In growth delay analysis, a statistically significant radiosensitization effect was observed for both tumors (p < 0.001 for both tumors). Difference for change in slope for control and valproic acid versus radiotherapy and radiotherapy plus valproic acid showed borderline significance for the U87MG cell line (p=0.065), indicating beyond additive effect, whereas this difference was statistically insignificant for A549 tumor (p=0.951), indicating additive effect.
ConclusionResults of this study indicate that a radiosensitizing effect for fractionated radiotherapy of valproic acid for A549 and U87MG tumors in vivo is evident and that it may be more than additive for U87MG tumors. Further exploitation of histone deacetylase inhibitors in clinical trials is warranted.
IntroductionHistone deacetylase (HDAC) inhibitor is considered a promising anti-cancer agent with an epigenetic modulation effect [1]. It also enhances the radiosensitivity of a variety of cancer cell lines [2-5].
Valproic acid (VA), originally an antiepileptic drug, has recently been shown to directly inhibit the enzymatic activity of HDAC [6], and subsequent in vitro and in vivo studies reported enhancement of radiosensitivity by VA in various cell lines [7,8]. Unlike other HDAC inhibitors (HDAC-I), such as sodium butyrate and trichostatin A, VA has several advantages, including good oral bioavailability and few demonstrated side effects [1]. Therefore, VA is a good candidate for clinical investigation as a radiosensitizer. However, in the aforementioned studies using VA, radiation was administered in a single fraction [7,8], whereas a fractionated regimen is usually employed in most clinical settings.
In this study, in vivo radiosensitization by VA was investigated in two human cancer cell lines, U87MG and A549, using fractionated radiation resembling that used in a clinical setting.
Materials and Methods1. Cell cultureA human lung cancer cell line, A549 (Korean Cell Line Bank, Seoul, Korea), was cultured in Dulbecco's Modified Eagle's medium (Welgene, Daegu, Korea) supplemented with 10% fetal bovine serum and 12.5 μg/mL of gentamicin. A human glioblastoma cell line, U87MG (Korean Cell Line Bank), was cultured at 37°C and 5% CO2 in culture media RPMI 1640 (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Gibco) and 12.5 μg/mL of gentamicin (Gibco).
2. Clonogenic assayCells were trypsinized from the exponentially growing monolayer cultures. The pre-determined numbers of cells were seeded into T25 flasks, followed by incubation for 24 hours prior to treatment. Combined cytotoxic effect of VA and radiation was compared with that of radiation alone. Both A549 and U87MG cells were exposed to 1.5 mM and 3 mM of VA. After exposure to VA for 18 hours prior to radiation, cells were irradiated using a 4-MV X-ray from a linear accelerator (Clinac 4/100, Varian Medical Systems, Palo Alto, CA) at a dose rate of 2.46 Gy/min. Graded radiation doses of 0, 2, 4, 6, and 8 Gy were used. After radiation, cells were incubated in drug free medium for 12 days for colony formation. The formed colonies were fixed with methanol and stained with 0.5% crystal violet; the number of colonies containing at least 50 cells was determined, and the surviving fraction was then calculated.
3. In vivo tumor modelA549 and U87MG cells, 5×106 in number, prepared in 15% fetal calf serum and 0.05 mL Waymouth’ media were administered by intradermal injection into the back of 6-week female BALB/c-nude mice (Orient, Seoul, Korea) weighing 15-25 g under anesthesia. Ketamine hydrochloride (Ketara, Yuhan Yanghang, Seoul, Korea) and xylazine hydrochloride (Rompun, Bayer Korea, Seoul, Korea) were mixed at a ratio of 5:1. Mixed solution was then diluted with normal saline at a ratio of 3:7. Prepared solution, 0.1 mL per 10 g weight of mice, was administered intraperitoneally to mice for pre-procedure anesthesia. Mice were then kept for a period of time until estimated tumor volume reached 250 mm3. Tumor volume was estimated using the formula (length× width×width)/2.
4. Growth delay assayTumor bearing mice were randomized into four groups; control, VA, irradiation (IR), and IR+VA, with eight mice in each group. Vehicle, which was phosphate buffered saline (PBS) in the current study, was administered intraperitoneally twice per day, 12 hours apart for 6 days for mice in the control group and the IR group. VA dissolved in PBS was administered intraperitoneally twice per day, 12 hours apart for 6 days for mice in the VA group and IR+VA group. Dosage used for VA was 150 mg/kg, mouse.
Irradiation was performed using a linear accelerator at a dose rate of 2.46 Gy/min. In the IR group and IR+VA group, 12 Gy in four fractions were delivered to the tumor harboring back of mice with a 1 cm bolus. Mice in the control group and VA group also underwent sham IR. Mice were irradiated for four consecutive days from the second day of administration of either vehicle or VA. The vehicle/VA and IR administration schedule is summarized in Fig. 1. To obtain growth curves, perpendicular diameters of each tumor were measured every 2-3 days using a digital caliper (Digimatic Caliper CD-15CPX, Mitutoyo Corporation, Kawasaki, Japan). Mice were euthanized using a CO chamber when the tumor volume exceeded 3,000 mm3.
The experiment was repeated three times for validation. In vivo experiments were approved by the Institutional Animal Care and Use Committee of Seoul National University Hospital, Clinical Research Institute (IACUC No. 06142).
5. Statistical analysisKaleidagraph ver. 3.51 (Synergy Software, Reading, PA) was used to fit the survival data of irradiated cells into a linear quadratic model. Chi-square test was used for statistical analysis of in vitro experiments. Dose enhancement factor (DEF), defined as ratio of dose with radiation alone over radiation with drug for the same biologic effect was calculated. SAS program ver. 9.1 (SAS Institute, Cary, NC) was used for statistical analysis of in vivo experiments. ANOVA was used to determine the effect of drug and radiation for in vivo experiments. Fisher's exact test for immunohistochemistry analysis was used for comparison of differences between groups. Relative growth delay was defined as difference in growth delay between two groups. In the current study, difference of growth delay between IR and IR+VA group was calculated to test the role of VA in addition to radiation. In addition, enhancement factor for growth delay, defined as ratio of days required for the tumor to grow to a certain volume for IR group over IR+VA group was calculated.
Probability values less than 0.05 were considered statistically significant and less than 0.1 were considered of borderline significance.
Results1. In vitro assaySurvival curves of A549 and U87MG cells treated with VA and radiation were compared with those of cells treated with radiation alone (Fig. 2). Radiosensitizing effect of VA on A549 cells was significant at a radiation dose greater than 4 Gy for 1.5 mM concentration and at every dose level for 3.0 mM concentration. However, for U87MG cells, radiosensitizing effect of VA was not evident at every dose level for 1.5 mM concentration and at doses greater than 4 Gy for 3.0 mM concentration. DEF at a surviving fraction of 0.3 for the A549 cell line was 1.31 and 1.35 for VA 1.5 mM and 3.0 mM, respectively. DEF at a surviving fraction of 0.1 for the U87MG cell line was 1.0 and 1.22 for VA 1.5 mM and 3.0 mM, respectively.
2. Growth delay assayTumor volume curves for A549 and U87MG cells for the control, VA, IR, and IR+VA groups are shown (Fig. 3). For A549 tumor, tumor growth delay was calculated at a tumor volume of 350 mm3. Tumor growth delays for the VA, IR, and IR+VA groups were 2.70±2.00 days, 6.33±3.62 days, and 11.07±1.62 days, respectively (p < 0.001). Relative growth delay for IR+VA group to IR group was 4.73±2.83 days. In addition, difference for change in slope of log values for control and VA group versus IR and IR+VA group was tested for beyond additive effect. Effect of IR and VA are more likely to be additive as it was statistically insignificant (p=0.951). For U87MG tumor, tumor growth delay was calculated at a tumor volume of 600 mm3. Tumor growth delays for the VA, IR, and IR+VA groups were 0.37±1.48 days, 3.30±3.20 days, and 7.0±2.78 days (p < 0.001). Relative growth delay for IR+VA group to IR group was 3.70±2.46 days. In addition, the effects of IR and VA are likely to be more than additive as difference for change in slope of log value for control and VA versus IR and IR+VA showed borderline significance (p=0.065). Enhancement factor for required days calculated for the same tumor volume was 0.77 and 0.74 for A549 and U87MG cells, respectively.
DiscussionIn recent decades, a new paradigm of "epigenetic changes", which are heritable changes in gene expression that are not caused by alterations in the gene nucleotide sequence, in relation to cancer development has evolved [9]. Histone modification is included in this epigenetic network as well as DNA methylation, chromatin remodeling, and microRNAs [10]. Unlike gene modulation, epigenetic processes are potentially reversible. DNA methylating agents and HDAC-I are the two most important classes of epigenetic drugs.
Effect of HDAC-I is exerted differently depending on the concentration. At a higher concentration, HDAC-I’s show tumor cytotoxicity. Mechanisms underlying this phenomenon are cell cycle redistribution, induction of apoptosis, and downregulation of surviving signals. However, radiosensitization effect is present at non-toxic low concentration and details of molecular mechanisms mediating radiosensitization by HDAC-I’s are less clear. It has been attributed, at least in part, to acetylation-induced changes leading to altered double strand break formation and repair [11]. Although some studies have reported possible increased radiosensitivity and reduced double strand break repair capacity in normal tissue cells and even teratogenicity of HDAC-I’s [12,13], there is also a contradictory study reporting a protective effect of HDAC-I’s [14,15].
Various types of HDAC-I’s have been identified so far. These can be grouped according to four categories; short-chain fatty acids, benzamides, hydroxamates, and cyclic peptides. VA is a short chain fatty acid which has long been used for treatment of epilepsy. Various cell lines, including human glioma, erythroleukemia, and colon cancer cell lines, have been tested for radiosensitizing effect of VA [7,8,16]. Unlike other HDAC-I’s such as sodium butyrate and trichostatin A, VA has a potential advantage of good oral bioavailability [1]. In addition, the antitumoral effects observed in preclinical studies were reached at concentrations that are readily achieved in patients treated with VA for epilepsy [17]. Therefore, VA is a suitable candidate for clinical investigation as a radiosensitizer.
In this study, in vitro and in vivo radiosensitization by VA was investigated in two human cancer cell lines, U87MG and A549, using fractionated radiation. These two cell lines, U87MG and A549, were chosen based on the background that glioma is the most frequent primary brain tumor with the highest malignant potential and that lung cancer is the most frequent site of origin for brain metastases, the most common form of brain tumor, also with dismal prognosis [18].
In in vitro study, radiosensitizing effect was more prominent for the A549 cell line, which showed a radiosensitizing effect at 1.5 mM above 4 Gy and even without radiation at 3.0 mM. This would mean that VA has a cytotoxic effect per se at 3.0 mM, whereas for U87MG, a radiosensitizing effect was only observed at 3.0 mM above 4 Gy. Camphausen et al. [7] reported a radiosensitizing effect of VA on other glioma cell lines, U251 and SF539. Concentrations of VA used for these cell lines were 1.5 mM for U251 and 2 mM for SF539, which is within a range similar to that of this study. The A549 cell line has been tested for radiosensitization effect using Vorinostat, SK-7041 and TSA [19-21], but not for VA.
Result from in vivo experiments in this study showed that VA has a radiosensitizing effect for tumors from A549 and U87MG cell lines. Other cell lines have been studied for radiosensitizing effect of VA, including the aforementioned U251 and LS174T [7,16]. However, in the aforementioned studies using VA, radiation was administered in a single fraction [7,8,16], whereas a fractionated regimen is usually employed in most clinical settings. Although single fraction radiotherapy in the form of radiosurgery is gaining ground, fractionated radiotherapy still plays a fundamental role in radiotherapy. Radiobiologic rationales for fractionated radiotherapy include repair, repopulation, reoxygenation, and redistribution [22]. Through fractionation, differential impact on tumor tissue and normal tissue is amplified. Thus, radiation tumor cytotoxicity is maximized in contrast to neighboring normal tissue toxicity.
Second aspect related to results from in vivo study is that radiosensitizing effect may be more than additive. Theoretically, the combination of HDAC-I and radiation could result in additive cell death due to different cytotoxic mechanisms associated with each modality. Both modalities could also act as more than additive because of the capacity of HDAC-I to modulate chromatin structure and to regulate gene expression [11].
Finally, study of the differential effect of combined treatment on both cell lines in in vitro and in vivo settings is needed. Radiosensitization effect of VA was evident even at lower concentration for the A549 cell line, whereas a relatively higher concentration was required for the U87MG cell line. On the contrary, growth delay in conjunction with radiotherapy was more evident for the U87MG cell line, whereas magnitude of difference was rather similar for the A549 cell line. Possible explanations for this finding are the growth potential and differential radiation dose response for respective cell lines. As shown in Fig. 3, average volume of control and VA group of U87MG cells were larger than 1,000 mm3 10 days after the grouping. However, average volume of the control group was less than 800 mm3 even after 20 days from the grouping. Radiation dose response was also different for the respective cell lines. The U87MG cell line showed continuous growth irrespective of group. On the contrary, in the A549 cell line, control and VA group showed continuous growth, whereas the IR and IR+VA groups showed volume reduction after treatment on day 4 and day 2, respectively. This finding indicates that radiation dose of 12Gy in four fractions was a cytoreductive dose only for the A549 cell line, but not for the U87MG cell line. Generally speaking, this difference of radiation sensitivity is not novel for these cell lines. Radiosensitivity, described as D0 for cells cultured in vitro, of sarcoma cell lines, which is often regarded as a radioresistant strain, is reported to be much higher, meaning radiosensitive, than that of squamous cell carcinoma cell lines, although variability exits [22]. Variability is explained in part through a variable proportion of cell killing mechanisms suggested by radiation. Where apoptosis plays an essential role in cell killing by radiation for more sensitive cell lines, apoptotic cell death is negligible for cell lines that are considered radioresistant. More importantly, difference in culture media for in vitro setting and surrounding environment, more often referred to as milieu, for an in vivo setting, where there may be a bystander effect, at least in part, due to modulation of various cytokines could be a cause of this difference. However, conduct of further studies investigating the underlying mechanism is required in order to validate this difference in an in vitro and in vivo setting.
Various HDAC-I’s have been tested in clinical trials. A pioneering study was a phase II study on the combination of VA, temozolomide, and radiotherapy (RT) for patients with high grade brain tumor. Currently, 11 studies utilizing VA and RT are listed on the http://www.clinicaltrials.gov site. Three studies are for brain tumor, one study for brain metastases, and the other studies are for various other solid tumors [23]. In our department, combination effect of VA on RT was tested retrospectively in glioblastoma patients [24]. Among 73 patients, 66 patients had VA administered during RT. Median survival for the VA (+) group was 25 months, while that for the VA (–) group was eight months (p=0.02). In multivariate analysis, adjusting for potential prognostic factors, including neurologic symptoms, extent of resection, and performance, VA administration showed statistical significance as an independent prognosticator for survival (p=0.02). Toxicity did not differ between the groups and no VA-related toxicity was observed.
ConclusionResult of this study indicate that a radiosensitizing effect for fractionated radiotherapy of VA for A549 and U87MG cells in vivo is evident and that it might be more than additive for U87MG cells. Conduct of further studies clarifying the underlying mechanism for cells in an in vitro and in vivo setting is warranted for better understanding of the radiosensitization potential of various HDAC-I’s. In addition, exploitation of HDAC-I’s in clinical trials based on findings from this in vivo study and retrospective study is warranted.
AcknowledgmentsThis study was in part supported by Seoul National University Hospital Research Fund (grant No. 04-2012-028-0), National R&D Program for Cancer Control by National Cancer Center Korea (grant No. 1320220), and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (No.2013M2A2A7043683).
Fig. 1.Summary of vehicle/valproic acid and radiation administration schedule. Vehicle, phosphate buffered saline; VA, valproic acid 150 mg/kg (mouse, intraperitoneal injection); IR, irradiation. 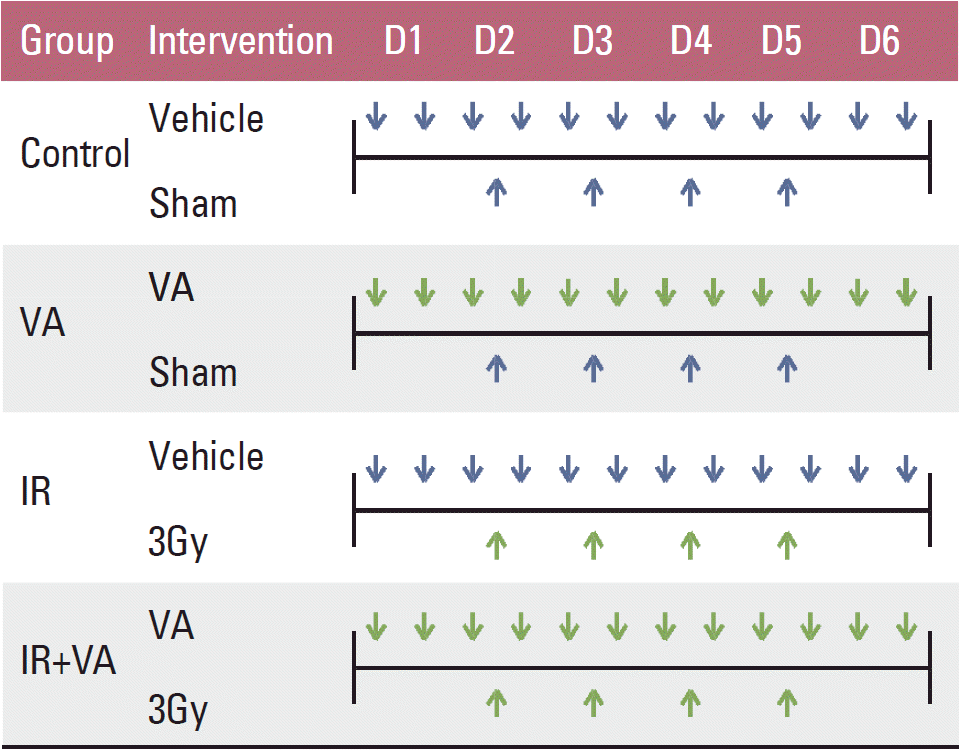
Fig. 2.Survival curves of A549 and U87MG cells treated with valproic acid at various concentrations. (A) A549 cell treated with valproic acid at 1.5 mM. (B) A549 cell treated with valproic acid at 3.0 mM. (C) U87MG cell treated with valproic acid at 1.5 mM. (D) U87MG cell treated with valproic acid at 3.0 mM. Points, mean for three independent experiments; bars, standard error. *p < 0.05, versus control. 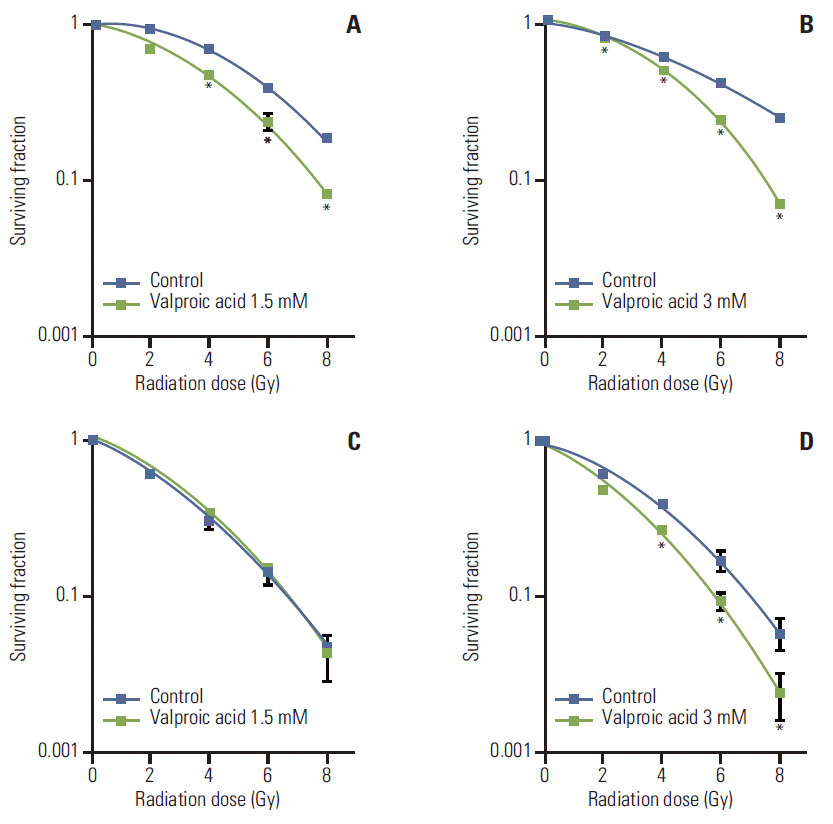
References1. Camphausen K, Tofilon PJ. Inhibition of histone deacetylation: a strategy for tumor radiosensitization. J Clin Oncol. 2007;25:4051–6.
2. Munshi A, Kurland JF, Nishikawa T, Tanaka T, Hobbs ML, Tucker SL, et al. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res. 2005;11:4912–22.
3. Kim JH, Shin JH, Kim IH. Susceptibility and radiosensitization of human glioblastoma cells to trichostatin A, a histone deacetylase inhibitor. Int J Radiat Oncol Biol Phys. 2004;59:1174–80.
4. Flatmark K, Nome RV, Folkvord S, Bratland A, Rasmussen H, Ellefsen MS, et al. Radiosensitization of colorectal carcinoma cell lines by histone deacetylase inhibition. Radiat Oncol. 2006;1:25.
5. Zhang Y, Jung M, Dritschilo A, Jung M. Enhancement of radiation sensitivity of human squamous carcinoma cells by histone deacetylase inhibitors. Radiat Res. 2004;161:667–74.
6. Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001;20:6969–78.
7. Camphausen K, Cerna D, Scott T, Sproull M, Burgan WE, Cerra MA, et al. Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer. 2005;114:380–6.
8. Karagiannis TC, Kn H, El-Osta A. The epigenetic modifier, valproic acid, enhances radiation sensitivity. Epigenetics. 2006;1:131–7.
9. Baylin SB, Ohm JE. Epigenetic gene silencing in cancer: a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–16.
10. Esteller M. Epigenetics provides a new generation of oncogenes and tumour-suppressor genes. Br J Cancer. 2006;94:179–83.
11. De Schutter H, Nuyts S. Radiosensitizing potential of epigenetic anticancer drugs. Anticancer Agents Med Chem. 2009;9:99–108.
12. Coyle TE, Bair AK, Stein C, Vajpayee N, Mehdi S, Wright J. Acute leukemia associated with valproic acid treatment: a novel mechanism for leukemogenesis? Am J Hematol. 2005;78:256–60.
13. Purrucker JC, Fricke A, Ong MF, Rube C, Rube CE, Mahlknecht U. HDAC inhibition radiosensitizes human normal tissue cells and reduces DNA double-strand break repair capacity. Oncol Rep. 2010;23:263–9.
14. Chung YL, Wang AJ, Yao LF. Antitumor histone deacetylase inhibitors suppress cutaneous radiation syndrome: implications for increasing therapeutic gain in cancer radiotherapy. Mol Cancer Ther. 2004;3:317–25.
15. Paoluzzi L, Figg WD. Histone deacetylase inhibitors are potent radiation protectants. Cancer Biol Ther. 2004;3:612–3.
16. Chen X, Wong P, Radany E, Wong JY. HDAC inhibitor, valproic acid, induces p53-dependent radiosensitization of colon cancer cells. Cancer Biother Radiopharm. 2009;24:689–99.
17. Driever PH, Knupfer MM, Cinatl J, Wolff JE. Valproic acid for the treatment of pediatric malignant glioma. Klin Padiatr. 1999;211:323–8.
18. Mehta MP, Buckner J, Sawaya RP, Cannon GM. Neoplasms of the central nervous systemIn : DeVita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg's cancer: principles and practice of oncology. 8th edPhiladelphia: Lippincott Williams and Wilkins; 2008. p. 1967–2032.
19. Munshi A, Tanaka T, Hobbs ML, Tucker SL, Richon VM, Meyn RE. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-H2AX foci. Mol Cancer Ther. 2006;5:1967–74.
20. Kim BK. Radiosensitizing effect and mechanisms of action of a novel HDAC inhibitor, SK-7041 [dissertation]. Seoul: Seoul National University; 2007. 19–25.
21. Kim IA, Kim IH, Kim HJ, Chie EK, Kim JS. HDAC inhibitor-mediated radiosensitization in human carcinoma cells: a general phenomenon? J Radiat Res. 2010;51:257–63.
22. Hall EJ, Giaccia AJ. Radiobiology for the radiologist. 6th edPhiladelphia: Lippincott Williams & Wilkins; 2006. p. 30-46, 378-97
23. U.S. National Institutes of Health. ClinicalTrials.gov. Search term “valproic acid” AND “radiotherapy” [Internet]. Bethesda: U.S. Department of Health and Human Services; c2013 [cited 2013 Oct 10]. Available from: http://www.clinicaltrials.gov/ct2/results?term=valproic+acid+radiotherapy
|
|
|||||||||||||||||||||||||||||||||||||||||||