INTRODUCTION
The most widely used agent for the treatment of metastatic colorectal cancer (CRC) is 5-fluorouracil (5-FU); it was developed for clinical use more than 40 years ago, and has been included in most regimens of chemotherapy for CRC (1). Until recently, the combination of 5-FU and leucovorin (LV) was considered to be the standard first-line treatment for patients with metastatic CRC. In patients with advanced CRC, treatment with 5-FU and LV reduces tumor size by 50 percent or more in approximately 20 percent of patients, and prolongs median survival from approximately six months (without treatment) to about 11 months (2).
Irinotecan is a semisynthetic derivative of the plant alkaloid camptothecin; it exerts its cytotoxic activity by inhibition of the nuclear enzyme topoisomerase I. Irinotecan has shown encouraging antitumor activity in a number of solid tumors, including colorectal cancer (3). As a first-line therapy, Rougier et al reported that 38 chemotherapy-naïve patients treated with 350 mg/m2 of irinotecan once every 3 weeks achieved on objective response rate of 18.8% (4). In addition, Ducreux et al reported in a phase I study that combined irinotecan with a bolus of 5-FU, continuous infusion of 5-FU and high dose leucovorin every 2 weeks, in previously treated metastatic colorectal cancer patients, produced a 22% response rate, with non-overlapping toxicity (5).
Oxaliplatin is a third generation platinum derivative that forms bulky DNA adducts and induces cellular apoptosis (6). Despite the ineffectiveness of other platinum-based agents such as cisplatin and carboplatin in the treatment of CRC, preclinical data from human cell lines has suggested that oxalplatin may be an option for treatment of this disease (7). The efficacy of oxaliplatin, as single agent, was evaluated in phase II studies demonstrating objective responses ranging from 20 to 24% in chemotherapy-naive patients with advanced CRC (8). In 5-FU-pretreated patients, oxaliplatin yielded a 10% objective response with additional disease stabilization of 24~42% (9). In irinotecan-pretreated patients with advanced CRC, oxaliplatin showed a 17% overall response rate with stable disease in 36% of cases (10). The toxicity profile of oxaliplatin was very mild, with a low incidence of myelosuppression, severe diarrhea and mucositis, and there were no cases of nephrotoxicity, ototoxicity or alopecia (11). The dose-limiting toxicity of oxaliplatin has been reported to be peripheral sensory neuropathy which is dose dependent and reversible (9).
In the present study, a phase II study was designed to evaluate the efficacy and safety of oxaliplatin in combination with a LV-modulated bolus plus infusion of 5-FU every 2 weeks in relapsed or advanced CRC patients who were previously treated with irinotecan-based combination chemotherapy.
MATERIALS AND METHODS
1) Eligibility criteria
Among patients with pathologically proven advanced or metastatic colorectal cancer, patients who showed progressive diseases after initial treatment of irinotecan combined with 5-FU and LV were enrolled. Other eligible criteria included: age (18~75 years); unidimensional measurable lesion; ECOG performance of at least 2; a life expectancy of at least 3 months; adequate hematological parameters (absolute neutrophil count ≥1,500/µl and platelets ≥100,000/µl); adequate hepatic (serum bilirubin ≤1.5 times the upper limit of normal; aspartate aminotransferase and alanine aminotransferase ≤5.0 times the upper limit of normal) and renal function (serum creatinine ≤1.5 mg/dl); absence of active infection or malnutrition; absence of a second primary tumor except for adequately treated in situ carcinoma of the cervix or a non-melanoma skin cancer. All patients gave their written informed consent before enrollment in the study.
2) Treatment schedule
The chemotherapy regimen consisted of oxaliplatin 85 mg/m2 on day 1; LV 200 mg/m2 on days 1 and 2; 5-FU 400 mg/m2 as an IV bolus, with 600 mg/m2 as a 22 hour intravenous infusion on days 1 and 2 repeated every 2 weeks. Treatment was continued until disease progression, unacceptable adverse effects or patient refusal.
3) Dose modification
Treatment was delayed after a maximum of 14 days. If there was a second episode of grade II or any grade III toxicities, the treatment was reduced 25%. If there was a third occurrence of grade II, a second episode of grade III or any grade IV toxicities, a 40% reduction was required. A fourth episode of grade II, a third episode of grade III or a second episode of grade IV toxicities, despite a dose reduction, resulted in treatment discontinuation. In the case of myelosuppression, the treatment was postponed or adjusted according to the following: in the case of a WBC <4,000/µl or platelets <100.000/µl at the start of a cycle, the treatment was postponed for one week. If after a maximum delay of 14 days, there was no full recovery, then the dose reduction of 25% or 40% was made as mentioned above.
4) Response criteria and toxicity
The pretreatment evaluation included a complete medical history and physical examination, complete blood count and chemistry profile measurements, a chest X-ray and a radiological tumor parameter assessment. Patients were assessed for their clinical response and classification was determined based on RECIST (Response Evaluation Criteria in Solid Tumors) every three cycles. Toxicities were assessed according to the National Cancer Institute (NCI) of Canada Clinical Trials Groups' expanded common toxicity grading version 2.0.
5) Statistical analysis
The end points were the overall objective tumor response rate, duration of response, progression free survival and overall survival. The progression free survival was calculated from the date of enrollment to the first observation of progressive disease or the occurrence of death from any cause. The response duration was calculated from the date of the first documented response to that of progression. All data were analyzed using SPSS version 12.0.1 for Windows. A survival curve was obtained using the Kaplan-Meier method.
RESULTS
1) Patient characteristics and treatment exposure
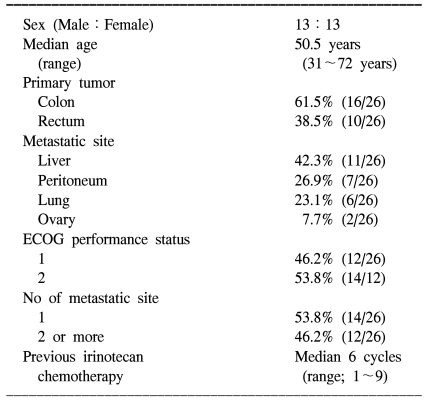
Between November 2002 and October 2005, 26 patients were enrolled in this study. Table 1 lists the baseline characteristics of all enrolled patients. One patient was excluded from the protocol because he refused to continue chemotherapy after one cycle. The mean dose intensity of oxaliplatin calculated during the treatment period was 39 mg/m2/week (92.2% of planned) and the median number of treatment cycles was 8 (range, 4~12 cycles). The mean dose intensity of 5-FU was 91.7% of the planned dose (917 mg/m2/week). In one patient, 5-FU was administered at 60% of the planned dose due to toxicity of a previous treatment. Patients who showed progressive disease after treatment received oral capecitabine for salvage therapy.
2) Efficacy
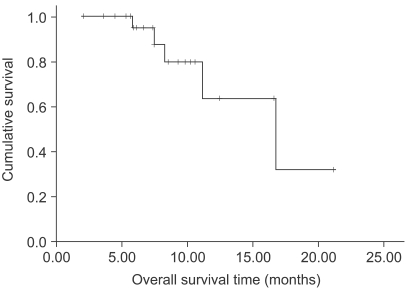
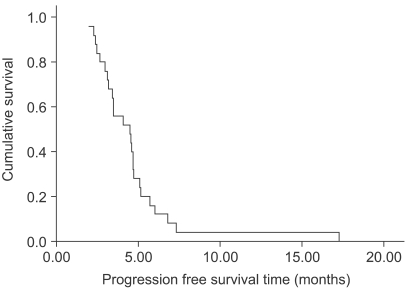
The response evaluation was assessed by an independent radiological review. A total of 25 patients were evaluated for efficacy. Four patients (16%) achieved partial responses and 15 (60%) had stable disease. However, no complete response was observed. The overall response rate was 16% (95% confidence interval; 1.7~30.3%). The follow-up duration of the surviving patients ranged from 2.0 months to 21.2 months. The median overall survival (OS) and 1-year OS rates were 16.7 months and 63.9% (Fig. 1). The median response duration and progression free survival were 2.8 (range: 1.6~4.7) and 4.5 (range: 2~17.3) months, respectively (Fig. 2).
3) Toxicity
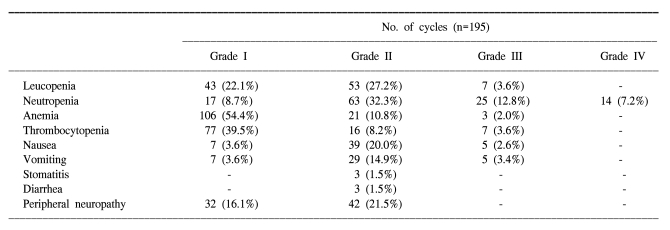
A total of 195 cycles were evaluated for toxicity, and the incidence of toxicity is summarized in Table 2. The most common hematological toxicities were NCI grade I/II leucopenia (49.3%), grade I/II neutropenia (41%) and grade I/II anemia (65.2%). Neutropenia reached grade III or IV in 39 cycles (20%). The main non- hematological toxicities were grade I/II peripheral neuropathy (16.1% and 21.5%, respectively) and nausea/vomiting (23.6%/18.5%). Grade II stomatitis and diarrhea developed in 3 cycles (1.5%), respectively. There was no life-threatening toxicity.
DISCUSSION
In a prior study, as a first-line chemotherapy in metastatic colorectal cancer, single-agent oxaliplatin produced a response rate, median time to progression (TTP), and survival time of 20% (95% confidence interval; 6.8~40.7%), 6 months and 14.5 months, respectively (12). Thus, single agent oxaliplatin has demonstrated an antitumor effect comparable to that of standard 5-FU and LV combination therapy. Furthermore, oxaliplatin has demonstrated promising antitumor activity in patients with 5-FU-refractory colon cancer, producing response rates of 10% (9).
Based on the promising single-agent activity of oxaliplatin for the treatment of colorectal cancer, its combination with different 5-FU-based regimens has been investigated. Interesting results, in terms of both efficacy and safety, have been reported for oxaliplatin in combination with a continuous infusion 5-FU/LV schedule as first- and second-line therapy (13,14). Two randomized phase III studies have confirmed the efficacy of oxaliplatin combined with the most frequently used 5-FU/ LV bolus and infusion regimens compared with the corresponding 5-FU/ LV alone. The combination therapy demonstrated a significant superiority in terms of efficacy including response rate, and median TTP, compared with 5-FU/LV alone (15,16). As a result, oxaliplatin in combination with either a bolus of 5-FU/ LV or an infusion regimen of 5-FU/LV has been approved as first-line treatment for patients with advanced or metastatic colorectal cancer, both in the United States and Europe.
In a recent study, the LV, 5-FU and irinotecan (FOLFIRI) protocol followed by the LV, 5-FU and oxaliplatin (FOLFOX 6) treatment showed a median survival and progression free survival, as a second line therapy, of 21.5 and 4.2 months, respectively (17). In another study, oxaliplatin, 5-FU and LV produced a significantly higher response rate and longer progression-free survival than 5-FU/FA in patients failing irinotecan-based therapies (18). These results suggested that the oxaliplatin based regimen was a useful second-line treatment for colorectal cancer.
In this study, we found that an oxaliplatin, 5-FU and LV combination chemotherapy regimen was an effective and safe treatment in relapsed or metastatic colorectal cancer patients as a second-line chemotherapy protocol. The overall response rate, time to progression and median survival time in our study were similar to those reported in previous studies (10,17). Although we used the FOLFOX 4 regimen, with a lower dose of oxaliplatin (85 mg/m2) than the FOLFOX6 (100 mg/m2) regimen, we observed a similar response rate and overall survival compared with a previous study (17).
The main safety concern regarding the use of oxaliplatin is peripheral neuropathy. Oxaliplatin induces frequent, transient, distal paresthesias during or shortly after the first minutes of infusion. In some cases these neurosensory symptoms increase in intensity with cumulative doses, persist between cycles and interfere with function (in the case of grade III effects) (14,16,11). Prior studies have reported grade III peripheral neuropathy in 12.4~34% of patients who received oxaliplatin (17,19,20). We observed grade I and II peripheral neuropathies in 17.6% and 21.6% of patients, respectively. We observed no grade III neuropathy. Our results show a lower incidence and milder grade of neuropathy than previous studies. This might be explained by the use of the FOLFOX 4 regimen, with its lower dose of oxaliplatin than the FOLFOX6 regimen, as well as the lower median number of treatment cycles than previously reported (20).
CONCLUSIONS
Although a relatively small number of patients were enrolled in this study, the results suggest that oxaliplatin, 5-FU and LV combination chemotherapy in a biweekly schedule is effective and well tolerated for the treatment of relapsed or metastatic colorectal cancer patients as a second line chemotherapy regimen.