INTRODUCTION
Colorectal cancer is common in the Western world, and a significant cause of death. In Eastern Europe, Japan and Korea, although the incidence of colorectal cancer is lower than that in Western countries, a sharp increase in cases has occurred (1~3).
Despite its increasing incidence, treatments for advanced colorectal cancer are not satisfactory. Currently, the ideal method for decreasing mortality from colorectal cancer is early detection of the malignancy (4,5).
Screening methods for colorectal cancer include the fecal occult blood test (FOBT), barium enema, sigmoidoscopy and colonoscopy. Large randomized controlled trials have shown that FOBT screening can result in decreased colorectal cancer mortality (6~8). However, using FOBT, the rates of false positive and false negative results are too high, and the sensitivities for detecting early stage colorectal cancer or adenoma are not satisfactory. Immunochemical modifications have recently been introduced to overcome these limitations to conventional guaiac-based FOBT (9,10).
Here, we report on a study designed to investigate the validity of a single immunochemical fecal occult blood test for the detection of colorectal neoplasia.
MATERIALS AND METHODS
Three thousand seven hundred and ninety four asymptomatic average-risk screenees, and three hundred and four colorectal cancer patients, admitted to the National Cancer Center, Korea, between May 2001 and November 2002 were studied prospectively. All screenees and colorectal cancer patients underwent FOBT and total colonoscopic examinations.
In the screenee group, all subjects visited the Center for Cancer Prevention & Detection, the National Cancer Center, Korea, for a medical check-up. The subjects with a previous colorectal pathology, such as colorectal cancers or polyps, and who had a family history of FAP or HNPCC were excluded. Subjects with recent colorectal symptoms, such as abdominal pain, diarrhea, constipation and hematochezia, were also excluded.
All three hundred and four colorectal cancer patients underwent colorectal resection surgery at the Center for Colorectal Cancer, the National Cancer Center, Korea. The tumor specimens were pathologically classified by T and Dukes stages (11).
For the total colonoscopic examination, the colonoscope was inserted as far as the cecum, with all the colorectal mucosae examined. Excluded from the study were cases where the colonoscope failed to reach the cecum and where poor bowel preparation prevented evaluation of the entire colorectal mucosae. All neoplasms detected during colonoscopy were biopsied or removed using the conventional polypectomy methods of snaring or hot biopsy, and all retrieved specimens examined pathologically.
High-risk adenomas were defined as adenomas with high grade dysplasia, a 10 mm or greater diameter or with at least 25% villous components.
All stools were collected by a conventional self-collection method prior to the colonoscopic examinations. Screenees and patients received a stool-collecting bottle, and collected stools by self-sampling. There were no dietary or medicinal restrictions. Stools were examined by immunochemical FOBT, using an OC-hemodia® (Eiken Chemical Co. Tokyo, Japan) and an OC-sensor analyzer® (Eiken Chemical Co. Tokyo, Japan). A positive FOBT result was defined as ≥100 ng/ml fecal hemoglobin.
The sensitivity of FOBT was defined as the positive rate of the FOBT in the group with colorectal adenomas or cancers confirmed by total colonoscopic examination. The specificity of FOBT in the screenees was defined as the negative rate of the FOBT in the group without colorectal neoplasia, as confirmed by the total colonoscopic examination. A false positive FOBT in the screenees was defined as a positive FOBT result, but with no colorectal adenoma or cancer detected by the total colonoscopic examination. A false negative FOBT in the screenees was defined as a negative FOBT result, but with colorectal neoplasia detected by the total colonoscopic examination. The positive predictive value of FOBT was defined as the probability that a person with a positive FOBT result had an adenoma or cancer according to the colonoscopy. The negative predictive value of FOBT was defined as the probability that a person with a negative test without an adenoma or cancer according to the colonoscopy.
Statistical significance was evaluated using the chi-squared test, with a two-tailed p value less than 0.05 defined as statistically significant.
RESULTS
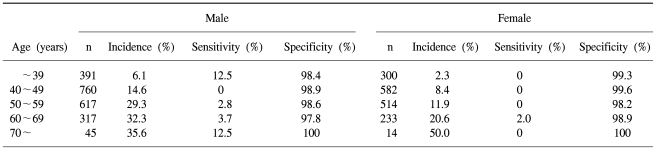
Of the three thousand seven hundred and ninety four asymptomatic average-risk screenees, 2151 were men and 1643 women, with a mean age of 48.9 years, ranging from 15 to 78 years (Table 1). The colonoscopies on the screenees showed 613 (16.2%) had colorectal adenomas and 67 (1.8%) high-risk adenomas. Colorectal cancers were detected in 12 (0.3%) screenees.
FOBT on these screenees showed a positive rate of 1.4%. The sensitivities of FOBT in detecting adenomas, high-risk adenomas and cancer were 2.4, 6.0 and 25.0%, respectively.
The false positive and negative rates of FOBT for colorectal adenomas or cancer were 1.19 and 97.6%, respectively. The positive and negative predictive values of the test were 28.3 and 84.0%, respectively.
The sensitivities and specificities of FOBT by gender and age of the screenees are listed in Table 2.
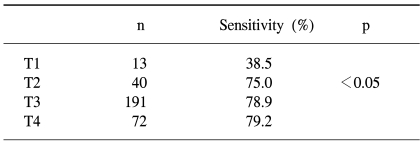
Of the three hundred and four colorectal cancer patients, 186 were men and 118 women, with a mean age of 59.6 years, ranging from 27 to 85 years (Table 1). The overall sensitivity of FOBT in these cases was 78.3%. Of the total 316 colorectal cancer cases (including the 12 cases from the screenees), the sensitivities according to T-stage were 38.5, 75.0, 78.9 and 79.2% for T1, 2, 3 and 4 cancers, respectively, with that for T1 tumors significantly lower than those for T2, 3 and 4 tumors (p<0.05) (Table 3).
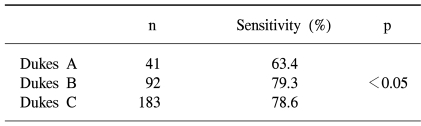
When classifying the tumors according to Dukes stage, the FOBT sensitivities were 63.4, and 78.6% for Dukes stages A, B and C, respectively, with that for Dukes A significantly lower than those for Dukes B and C (p<0.05) (Table 4).
DISCUSSION
Screening asymptomatic people for colorectal cancer can reduce mortality from this disease. Many experts recommend various screening tools for the average-risk population over 50 years of age, namely FOBT, sigmoidoscopy, barium enema and colonoscopy (12,13). Among these screening tools, FOBT has been shown to be effective in reducing mortality from colon cancer in large randomized controlled trials, and is relatively cheap, easy to perform and non-invasive (6~8,14). However, FOBT is known to have low sensitivities for early stage colorectal cancer and adenomas. Several modifications have been attempted in order to overcome the deficiencies of the FOBT, including guaiac methods, with rehydration techniques and immunochemical methods (9,10,15).
Lieberman and Weiss (16) reported the sensitivity for the fecal occult blood test alone, for any neoplasia, was 11.7%, with that for advanced neoplasia (adenoma 10 mm or greater in diameter, at least 25% villous, high grade dysplasia or a classified invasive cancer) was 23.9%. They used a method involving guaiac-impregnated cards (Hemoccult II) and rehydration. Nakama et al. (17) reported the sensitivities for the one-day immunochemical FOBT for colorectal cancer and adenomas were 55.6 and 30.1% respectively. They also reported the sensitivities for colorectal cancer were 83.3 and 88.9% for the two- and three-day methods, respectively (17). Although the sensitivity of FOBT was higher than with the two- or three-day methods used in previous studies, we investigated the diagnostic validity of FOBT using the one-day method, which is convenient and widely used for colorectal cancer screening in Korea.
In our study, we used an immunochemical FOBT, which is more sensitive than conventional guaiac-based FOBT (18). Our data show a single immunochemical FOBT in asymptomatic average-risk screenees had sensitivities of 25.0 and 2.4% for colorectal cancer and colorectal adenomas, respectively. The sensitivities for colorectal cancer and adenomas in our study were lower than those in previous studies (9,10,15~18). This may have been because in the present study, total colonoscopies and FOBT were performed on all asymptomatic screenees, which led to a greater detection rate of early stage colorectal cancer. However, from our results, the sensitivity for colorectal cancer in the admitted group was 78.3%, which was not much different from the other studies showing the sensitivity of immunochemical FOBT for colorectal cancer (19). Also, of the 12 detected colorectal cancer cases in the asymptomatic average-risk screenee group, 4 (30%) were identified with Dukes stage A cancers, whereas 37 (12.2%) were identified to Dukes stage A cancers in the 304 admitted colorectal cancer patients, and this difference was found to be statistically significant (p<0.05).
The sensitivity and specificity of the fecal occult blood test are affected by the cut-off values and fecal collection time. Physiologic blood loss from the gastrointestinal tract has been estimated to be 0.32±0.09 mg/g stool (20). Nakama et al. (21) reported that 150 ng/ml of fecal hemoglobin was the optimal cut-off point when carrying out the OC-hemodia test as a means of screening for colorectal cancer, but no significant difference was observed in the specificities between 50 and 150 ng/ml. In our study, a positive FOBT result was defined as ≥ 100 ng/ml fecal hemoglobin. When using this lower cut-off point, the sensitivity of FOBT can be increased. However, the optimal cut-off point must be calculated by the positive predictive value, receiver operating characteristic (ROC) curve and from a cost-effectiveness analysis. Further study is required to evaluate the cost-effectiveness and validity of immunochemical FOBT.
Early detection of early stage colorectal cancer has recently been emphasized as the ideal method for increasing the survival rates and quality of life. Despite the importance of research for early stage colorectal cancer detection, it has largely been limited to colonoscopic diagnosis and management. In the National Polyp Study (22), patients who underwent colonoscopy and removal of all polyps had a lower incidence of colorectal cancer during six years of follow-up than reference populations. There is no doubt that colonoscopy is the best method for detecting colorectal cancer or adenomas, and a synchronous colorectal pathology (22,23). However, this procedure is invasive, expensive and requires skillful endoscopists (14,24). In addition, it has not yet been proven that a colonoscopic examination reduces the rate of mortality from colorectal cancer any more than other screening tools.
Of the current screening tools for colorectal cancer, FOBT has been proved to reduce the mortality from colorectal cancer in large randomized controlled trials (6~8). However, the present study indicates a single immunochemical FOBT is not sufficient for detection of colorectal cancer in screenees. Further work will be required in order to identify the ideal screening tools for detection of colorectal cancer in order to decrease the mortality from this disease.
CONCLUSIONS
The sensitivities of a single immunochemical FOBT for detecting colorectal cancers and adenomas in screenees were 25.0 and 2.4%, respectively. The sensitivities according to Dukes stage were 63.4, 79.3 and 78.6% for Dukes A, B and C, respectively. These findings indicate that about one-third of Dukes A colorectal cancers appear to be undetected by a single immunochemical FOBT screen.