AbstractPurposeTo analyze the radioresponse of hepatocellular carcinomas (HCC), using accurate measurements of the tumor size in extrahepatic lymph node metastasis, and to obtain information for the future treatment of primary intrahepatic lesions.
Materials and MethodsFifty-one extrahepatic lymph node metastases from primary HCCs, which could be treated by external radiotherapy alone, were included in this study. The radiation dose ranged from 30 to 51 Gy with fraction sizes of 2.0~3.0 Gy. Responses were determined by measuring the areas on CT scans 0, 1 and 3 months after the completion of radiotherapy. The median follow-up period of the surviving patients was 10 months.
ResultsThe overall response rate was 76%, and the important factors were; total dose of radiation, time dose fractionation (TDF) value and the biologically effective dose (BED). A dose of 45 Gy or higher showed an objective response rate of 93%, and if the TDF value was higher than 90, a similar result was observed. In about half (47%) of the patients the maximum response was observed at 3 months or later. The response duration was observable in 14 patients surviving 12 months or longer. Regrowth of irradiated lesions were observed in 4 (66.7%) patients among those who received less than 45 Gy, and in 4 (50%) among those who were treated with 45 Gy or more. There was a statistically significant difference in the survivals between the responders and non-responders (p=0.008). Gastrointestinal bleeding or ulceration was observed in 8 patients, including 3 with NCI common toxicity criteria grade III or higher.
INTRODUCTIONHepatocellular carcinomas (HCC) are one of the most common cancers in Asia, because of the prevalence of chronic liver disease due to HBV infection, and patients with HCC usually have a poor prognosis. For the treatment of HCC, surgical resection after early diagnosis has been the only curative mode of treatment. However, the majority of HCC's are diagnosed in unresectable states (1), and characteristics, such as multifocality, vascular invasion and accompanying chronic liver diseases, make the complete resection of tumors difficult. Unresectable lesions have been treated using various targeted therapies, such as transarterial chemoembolization (TACE), radiofrequency (RF), percutaneous ethanol injection (PEI), radioimmunotherapy and hot saline injection (HSI). On the other hand, the role of radiotherapy in HCC has been largely palliative (1,2). Some investigators have reported excellent results after radiotherapy alone (3) or combined with TACE (4,5), though the response evaluation after TACE might not be easy as it is difficult to accurately measure the size of a viable tumor.
In cases of lymph node metastases from HCC, there is no effective treatment. Local injection therapy is not feasible due to the location of the bowels in front of the nodes, and the impossibility of applying chemoembolization. Systemic chemotherapy has produced disappointing results (6). Therefore, extrahepatic lymph node metastases of HCC have been treated for several years by radiotherapy alone to achieve the salvage of the diseases or symptoms palliation. The treatment results were retrospectively reviewed to analyze the radiation effect on these patients, to evaluate treatment-related toxicities and to obtain information for the future treatment of primary intrahepatic lesions.
MATERIALS AND METHODS1) Patients and treatmentBetween May 1, 1997 and May 31, 2001, 51 patients were treated for extrahepatic lymph node metastases from primary HCC that could be treated by external radiotherapy alone (Table 1). Most of the lesions (48/51) were located in the upper abdomen (at porta hepatis, portocaval, aortocaval or paraaortic areas) and three patients had node metastases beyond the abdominal cavity. The number of enlarged nodes in some patients could not be exactly determined as they were in a conglomerated state, but the size of those conglomerated, as a whole, could be measured exactly. In 37 (73%) of the patients, the aim of the treatment was symptom palliation, and the others (27%) were treated with curative intents as their intrahepatic lesions were well controlled with other treatment modalities. Informed consent was obtained from every patient.
A CT simulator (AcQSim, Picker, OH) was used to localize the involved lymph nodes, liver, both kidneys and spinal cord. The radiation field included nodes with lateral margins of 1 or 2 cm and superior and inferior margins of 3 cm. Every treatment plan was made by the co-planar method (Render plan, Elekta, Sweden) and the dose-volume histograms (DVH's) of the liver and kidneys were calculated to limit excessive exposure.
However, a portion or the whole circumferences of the duodenum and/or the gastric antrum were inevitably included during the treatment of the paraaortic or portocaval lymph nodes. Although 30 Gy, in 10 Gy fractions, was the base treatment scheme used for palliative purposes during the early phase of this study, the dose was increased gradually up to 51 Gy, in 17 Gy fractions, to achieve a better result in 7 patients who were treated with curative intents. However, 45 or 50 Gy in 15 or 25 Gy fractions, respectively, became the standard regimen after ulcer bleeding had occurred at the gastric antrum occurred in two cases.
2) Response and survival evaluationResponses were determined by measuring the largest area on the CT scans checked immediately after the completion of radiotherapy, and at 1, 3 and 6 months after the treatment. A complete response (CR) was defined as complete disappearance of the lesion within the radiation field, while a partial response (PR) was defined as a decrease in the tumor area by more than 50% of the initial size, and a minimal response (MR) as shrinkage from 10 to 50% of the initial area. Differences in response rates between the dose groups were compared using the Fisher's exact test (2-tailed) and the dose-response relationship with a likelihood ratio test for trends. The survival time was measured from the date of first radiotherapy to the date of death or the last follow-up. The overall survival rate was determined using the Kaplan-Meier method, and the comparison of survival rates was made with the log-rank test, with Cox regression performed to identify the independent variables for predicting survival.
3) Toxicity evaluationNCI common toxicity criteria (7) were used for grading complications, and were as follows; grade II, requiring medical management or non-surgical treatment; grade III, bleeding without perforation, uncontrollable by outpatient medical management or requiring hospitalization; grade IV, perforation or bleeding requiring emergency surgery.
Antacid and H2-antagonist were used to reduce the risk of radiation-induced gastroduodenitis or ulceration in the radiation field. CBC and liver function tests were checked weekly during radiotherapy, and duodenofibroscopy was performed on any symptom suggestive of gastritis or bleeding. The median follow-up period of the surviving patients was 10 months and the minimum follow-up was 6 months.
RESULTSTreatment was well tolerated in all patients, with the exception of one who stopped treatment for personal reasons. No patient complained of upper gastrointestinal symptoms related to the radiotherapy during treatment.
Response evaluation at 3 months or later was performed in 42 patients (82%), but in 7 patients, response evaluations were done at 0 and 1 month due to early death before 3 months. Two patients were lost to follow-up after 3 months.
The overall response rate was 76%, and the radiation dose was critical to the response (Table 2). For total doses of 45 Gy or higher, 26 of the 28 patients (93%) showed major responses in contrast to 13 of the 23 patients (57%) who were treated with less than 45 Gy (p=0.003). Moreover, a definite dose-response relationship was found from the likelihood ratio test for trends (p=0.002).
The time dose fractionation (TDF) value was also an important factor in the tumor response. Patients who received a dose of less than TDF 90 showed poorer responses than those that received a TDF 90 or more (58% vs. 93%, p=0.007) (Table 3). When the response rates were compared according to the BED, with various/ratios, the results were almost the same as those of the total dose and TDF value (Table 4).
Smaller tumors tended to show a better response, although this was not significant (Table 5). In particular, tumors less than 20 cm2 showed a much higher CR rate (67%) than larger tumors (17%).
The times to a maximum response was evaluated among the 34 major responders who had received a follow-up CT scan at more than 3 months after treatment. About half of the patients (47%) showed a maximum response at 3 months or later, and the slowest response occurred at 12 months.
The response duration could be analyzed only in 14 patients who survived 12 months or longer. In-field recurrence was observed in 8 patients and regrowth of irradiated lesions in 4 (66.7%) who received less than 45 Gy and in a further 4 (50%) who were treated with 45 Gy or more. The median response duration of each treatment group was 8 (range; 6~21) and 9 months (range; 5~38), respectively.
The survival rates were evaluated in all 51 patients, and were 31.3, 15.7 and 8.7% at 1, 2 and 3 years, respectively, with a median survival of 7 months (Fig. 1). The survival rate of the responders was significantly higher that that of the non-responders. The two-year survival rate for the 39 responders was 20.5%, whereas none of the patients obtaining neither CR nor PR survived for 2 years (p=0.008) (Fig. 2). The treatment aim also affected the survival rates, despite showing no significant relation to the radiation response rate. The two-year survival rates of those who were treated with curative and palliative aims were 46.2 and 0%, respectively (p=0.0001). A multivariate analysis revealed that the radiation response and treatment aims were significant prognostic factors for survival.
Upper GI bleeding was the most common complication, and observed in 8 patients. These were largely controlled with conservative treatment, with the exception of 3 patients who needed hospitalization. One of these received a distal subtotal gastrectomy and another expired due to uncontrolled bleeding. However, the relationship between the incidence of ulcer bleeding and radiation dose was not certain, as bleeding was observed in 3 of the 8 patients (38%) who were treated with 40 Gy or less, and in 5 of the 13 (38%) treated with more than 40 Gy. A history of chronic gastritis or ulceration, with the inclusion of the whole circumference of the gastric antrum, was an important factor for the development of bleeding.
DISCUSSIONThe role of radiotherapy in treating HCC was limited to the palliative aim because of the radioresistance and low tolerance of the liver to radiation. As a result, high dose radiotherapy has not been feasible in most cases, which in turn has made it impossible to obtain outstanding responses (8,9). Recently, many investigators have reported high response rates with external beam radiation using sophisticated radiotherapy, such as 3-D conformal or proton beam treatments (4,5,8,10~13), which have proven that HCC are not radioresistant (Table 6). It is noteworthy that larger fraction sizes (≥2.5 Gy) or daily doses higher than the conventional dose showed higher response rates than conventional regimens (10,12,13). The reason for these high response rates is not clearly understood, but one can presume that HCC cell lines may have a lower α/β ratio than expected. As the α/β ratio of HCC cell lines are not well understood, the BED was calculated using various α/β ratios (1.5, 3 and 10), and dose-response relationships were observed with every ratio. However, as shown in Michigan group's report, two daily fractions of 1.5 Gy produced excellent results if a sufficient dose (higher than 60 Gy) was delivered, with concurrent chemotherapy as a radiosensitizer. This means the total dose would be more important than the fraction size in treating HCC, and may be an important reason for the low response rate of palliative radiation with doses around 30 Gy in 10 Gy fractions. In the present study, HCC were confirmed to be radioresponsive and curable if sufficient radiation was delivered. Although the doses required to achieve complete tumor control were not established in this study, it was found that major responses could be obtained with 45 Gy, in 3 Gy fractions, or with TDF 90, which was similar to the results reported by Kaizu and Nagashima (14,15). It can also be concluded that doses of 45 Gy seemed to be enough for patients whose life expectancy was lower than 1 year, as only one patient survived more than one year after treatment and the others died due to progression of the primary intrahepatic tumors and aggravation of the hepatic function.
Although radiation doses around 45 Gy were effective for major responses, these were insufficient to eradicate tumors because there were 10 cases of re-growing lesions within the radiation field. An adequate dose range could not be determined for the eradication of HCC, as a dose beyond 51 Gy could not be used for fear of gastrointestinal complications. It is our belief it would be possible to determine an adequate dose range for tumor eradication, with higher total doses and by treating primary HCC located at a substantial distance from the bowels. Another reason the response duration could not be determined is that almost all of our patients died of chronic liver disease and/or advanced intrahepatic lesions, making our observation of tumor recurrence incomplete. However, if they survived for a longer period of time, it would be very probable that a higher number of tumor regrowths would have been observed.
The overall survival rate in this study was disappointing, due to the uncontrolled primary intrahepatic lesions (37 patients, 72.5%), and all of our patients had extrahepatic lymph node involvement, which were distant metastases. Although the treatment of lymph node metastasis did not change the overall disease progression in our subset of patients, the curative intent of treatment and major response were observed to have an influence on better survival. On the basis of this observation, it could assume that if the intrahepatic lesions were in controlled or stable states, and the patients had had decent hepatic functions, the survival rate could have been higher than observed. However, it would be necessary to apply strict eligibility criteria to show such a positive effect of radiation, which seemed to make no sense with the patients in the present study.
Gastrointestinal bleeding occurred between 1 and 2 months after the completion of radiotherapy, although the patients complained of no ulcer symptoms. The areas of bleeding were well correlated with the radiation fields on duodenofibroscopy.
The important factors of bleeding were considered to be a history of gastroduodenitis before treatment, inclusion of the whole circumference of the gastric antrum and the radiation dose. A decreased hepatic function, with low platelet counts, might be a reason for bleeding, but this is not likely as these were within the normal range in the patients concerned. The most probable reason was preexisting erosive gastritis or an ulcer, as all of our bleeding cases had mild gastritis before irradiation. The treatment technique was also important as bleeding occurred in patients who were treated initially with AP-PA parallel opposing fields of up to 30 Gy (which included the whole circumference of the gastric antrum), and then with anterior-right-left 3 fields to total doses from 45 to 51 Gy. Subsequently, the AP-PA POP field was changed to the 3 or 4 field technique to avoid irradiating the whole circumference of the antrum from the beginning of treatment; grade 3 or higher bleeding was not observed. In addition, a dose of 51 Gy was too high if the gastric antrum was included in the radiation field. Two patients were treated with 51 Gy, and both suffered serious bleeding. It is usually accepted that the complication probabilities of 5% (TD5/5) and 50% (TD50/5) of the whole stomach are at 50 and 60 Gy, respectively, for whole organ irradiation with 2 Gy fractions (16). The TD5/5 and TD50/5 were set at 60 and 70 Gy, respectively, with conventional fractionation, if the irradiated volume of the stomach was less than one third of the entire organ (17). In the present study, the volumes of the stomach or duodenum included in radiation field were less than 1/3 of the whole organ, and doses were within the ranges mentioned above. A TD5/5 at 45 Gy, in 3 Gy fractions, was equivalent to the TDF value of 56 Gy, in 2 Gy fractions, and to 48.75 Gy, in 2 Gy fractions, if the α/β ratio was 10 Gy for the stomach and duodenum. It is recommended that the dose TD5/5 be reduced for the treatment of HCC patients, especially in those with gastritis or an ulcer at the time of diagnosis. Currently, antacid and H2-antagonists are used during and for 1 month after irradiation. Moreover, it is not our belief that TDF and BED represent a perfect conversion of the fractionation schedule for normal organs, although they are widely used to compare fractionation schedules.
CONCLUSIONSThis study has shown that hepatocellular carcinomas are radioresponsive tumors, and doses of 45 Gy or higher (TDF 90 or higher) are required to achieve a major response in the treatment of extrahepatic lymph node metastases. A good response would also have promising results on survival. Although the optimal dose for cure could not be defined, radiotherapy was found to be a good alternative for treatment of unresectable intrahepatic HCC if the gastrointestinal tract could be precluded from the radiation field.
References1. Dusheiko GM, Hobbs KE, Dick R, Burroughs AK. Treatment of small hepatocellular carcinomas. Lancet. 1992;340:285–288. PMID: 1353202
2. Liu CL, Fan ST. Nonresectional therapies for hepatocellular carcinoma. Am J Surg. 1997;173:358–365. PMID: 9136797
3. Kim DY, Lee JH, Koh KC, Paik SW, Ahn YC, Huh SJ, Yeo IJ, Park SW, Chang SH. Dose-response relationship of radiotherapy for locally advanced hepatocellular carcinoma. J Korean Cancer Assoc. 2000;32:918–924.
4. Guo WJ, Yu EX. Evaluation of combined therapy with chemoembolization and irradiation for large hepatocellular carcinoma. Br J Radiol. 2000;73:1091–1097. PMID: 11271902
5. Seong J, Park HC, Han KH, Lee DY, Lee JT, Chon CY, Moon YM, Suh CO. Local radiotherapy for unresectable hepatocellular carcinoma patients who failed with transcatheter arterial chemoembolization. Int J Radiat Oncol Biol Phys. 2000;47:1331–1335. PMID: 10889387
6. Farmer DG, Rosove MH, Shaked A, Busuttil RW. Current treatment modalities for hepatocellular carcinoma. Ann Surg. 1994;219:236–247. PMID: 8147605
7. Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, Gunderson L, McCormick B, Morrisintegral M, Rich T, Shipley W, Curran W. Common toxicity criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:13–47. PMID: 10758303
8. Stillwagon GB, Order SE, Guse C, Leibel SA, Asbell SO, Klein JL, Leichner PK. Prognostic factors in unresectable hepatocellular cancer: Radiation Therapy Oncology Group Study 83-01. Int J Radiat Oncol Biol Phys. 1991;20:65–71. PMID: 1847127
9. Abrams RA, Cardinale RM, Enger C, Haulk TL, Hurwitz H, Osterman F, Sitzmann JV. Influence of prognostic groupings and treatment results in the management of unresectable hepatoma: Experience with cisplatinum-based chemoradiotherapy in 76 patients. Int J Radiat Oncol Biol Phys. 1997;39:1077–1085. PMID: 9392547
10. Blomgren H, Lax I, Granson H, Krpelien T, Nilsson B, Nslund I, Svanstrm R, Tilikidis A. Radiosurgery for tumors in the body: Clinical experience using a new method. J Radiosurg. 1998;1:63–75.
11. Matsuura M, Nakajima N, Arai K, Ito K. The usefulness of radiation therapy for hepatocellular carcinoma. Hepatogastroenterology. 1998;45:791–796. PMID: 9684136
12. Matsuzaki Y, Osuga T, Saito Y, Chuganji Y, Tanaka N, Shoda J, Tsuji H, Tsujii H. A new, effective, and safe therapeutic option using proton irradiation for hepatocellular carcinoma. Gastroenterology. 1994;106:1032–1041. PMID: 7511552
13. Robertson JM, Lawrence TS, Andrews JC, Walker S, Kessler ML, Ensminger WD. Long-term results of hepatic artery fluorodeoxyuridine and conformal radiation therapy for primary for primary hepatobiliary cancers. Int J Radiat Oncol Biol Phys. 1997;37:325–330. PMID: 9069303
14. Kaizu T, Karasawa K, Tanaka Y, Matuda T, Kurosaki H, Tanaka S, Kumazaki T. Radiotherapy for osseous metastases from hepatocellular carcinoma: a retrospective study of 57 patients. Am J Gastroenterol. 1998;93:2167–2171. PMID: 9820391
15. Nagashima T. The study on radiotherapy for hepatocellular carcinoma. Nippon Igaku Hoshasen Gakkai Zasshi. 1989;49:1141–1151. PMID: 2555769
16. Rubin P, Constine LS, Williams JP. In : Perez CA, Brady LW, editors. Late effects of cancer treatment: Radiation and drug toxicity. Principles and practice of radiation oncology. 1998. 3rd edPhiladelphia: Lippincott Williams & Wilkins; p. 143–154.
17. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, Shank B, Solin LJ, Wesson M. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. PMID: 2032882
Fig. 1Overall survival curve for 51 patients. Survival rates at 1, 2 and 3 years were 31.3, 15.7 and 8.7%, respectively, with a median survival time of 7 months. 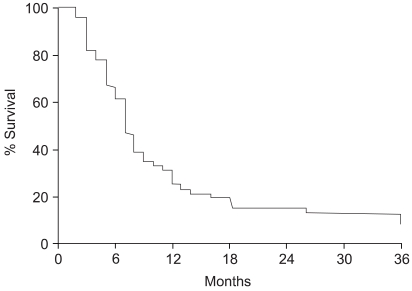
Fig. 2Survival curves in relation to the radiation response. The two-year survival rate for the 39 patients who achieved either CR or PR after radiation therapy was 20.5%, whereas none of the patients who obtained neither CR nor PR survived for 2 years (p=0.008). 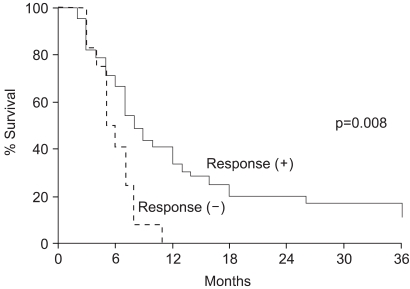
|
|
||||||||||||||||||||||||||||||||||||